Biofunctionalization of surfaces by covalent co-immobilization of RGD and PHSRN-containing oligopeptides
Biofunctionalization of surfaces by covalent co-immobilization of RGD and PHSRN-containing oligopeptides
Surface functionalization of biomedical materials provides materials with specific biological activities. This is relevant to the implant industry in the design and modification of implant surfaces in a way conducive to tissue regeneration and accelerating the healing process.
Titanium (Ti) is a material often used for the construction of dental and orthopedic implants, because of its excellent mechanical characteristics, chemical stability, and biocompatibility [1]. However, because titanium is an inert material, and as such cannot encourage cell growth on and around the implant site, titanium implants may hinder bone formation [2]. Another problem, which similarly impacts the healing process, is infections at the implant site [3,4].
One of the methods used to try and overcome these medical problems is the functionalization of the titanium surfaces by coatings the implant with materials containing molecules with known biological activities [5,6]. A variety of molecules have been used in recent years to coat Ti surfaces, including bioactive proteins (Bone Morphogenic Proteins (BMPs), collagen) [7-10], nucleotides [11,12], peptides (RGD, FNIII [7-10]) [13-17]), and antimicrobial agents [18-22], although presently molecules must be coated individually. The next challenge in implant coating technology will be coating the same surface with multiple materials that will contribute to a variety of surface bioactivities, including mineral formation, cell recruitment, cell differentiation and proliferation, improvement of osseointegration, and antimicrobial activities; in order to do that, many factors must be explored, such as electrostatic interactions and physical absorption of the different molecules that are used to coat the Ti surface [23]. This research has to lead to promising coating techniques, such as hyaluronic acid/chitosan polyelectrolyte multilayer combined with RGD peptides, which inhibits bacterial adhesion and growth and promotes bone cell functions [24]. Another example is a Ti surface bearing BMP-2 and gentamicin, which enhances osseointegration, combined with antibacterial properties [25].
A major problem arises regarding implant coating stability. Physical absorption and electrostatic attraction, for example, may not be suitable methods for producing coatings that are stable enough to remain functional after surgical scenarios or in the biological environment around the implantation site. A coating technique with promising results is coating by covalent bonding between the Ti and the molecules. Methods for biomechanical immobilization, such as coupling with silane agents [16,26] or thiols [27,28], were shown to produce surfaces with strong mechanical and biomechanical stability.
In the 2013 article “Surface biofunctionalization by covalent co-immobilization of Oligopeptides” [29], Chen et al present a simple and reliable oligopeptide coating method using covalent conjugation to co-immobilize two oligopeptides known for their cooperative bioactivities: PHSRN and RGD. While PHSRN is not biologically active by itself, it has a synergistic effect with RGD, as these two molecules cooperate in the construction of fibronectin [30,31]. Recombinant peptides, which include motifs from these two peptides [32-36], and multi-peptide systems including PHSRN and RGD [31], were shown to increase cell adhesion, proliferation, and expansion, in comparison to systems that use the RGD motif only. In their study, Chen et al incorporated peptides that contain PHSRN and RGD motifs onto Ti surfaces, using organosilanes for covalent linking. The synergistic effect on cell response of these two peptides was tested on model surfaces, and the ability of these modified Ti surfaces to retain the biological characteristics of the immobilized peptides was explored.
The oligopeptides described in the article were attached to the surface in a three-step method using CPTES as a coupling agent to connect the peptides to the Ti surface. As Figure 1, taken from that article, depicts, there is an even distribution of the PHSRN and RGD molecules; therefore, it can be seen that the co-immobilizing process was successful.
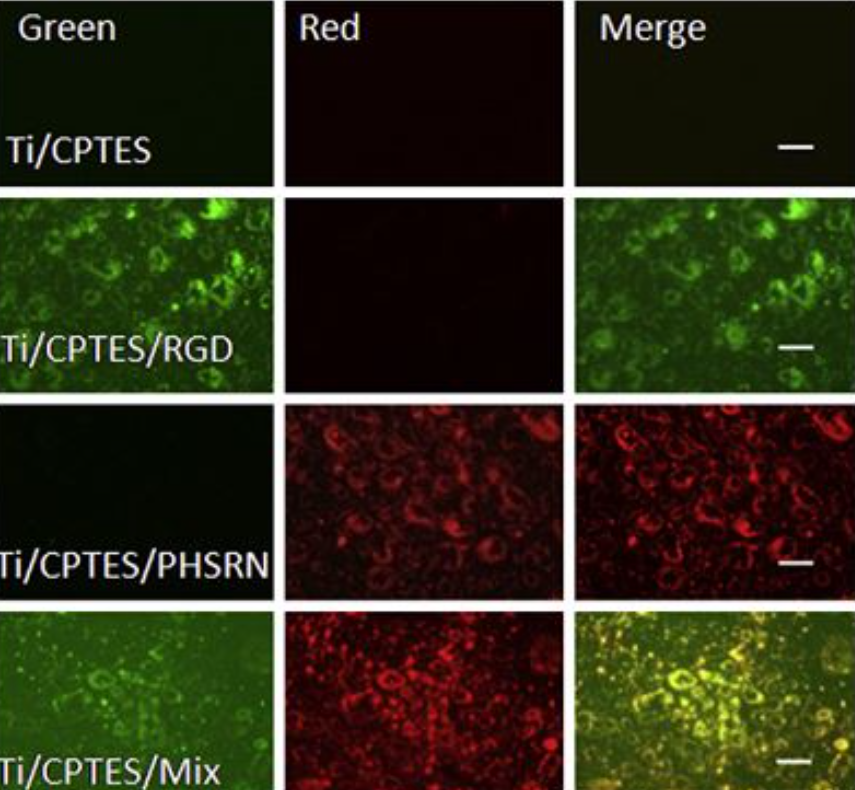
Figure 1: Co-immobilization of PHSRN and RGD.
PHSRN-containing and RGD-containing peptides were conjugated to red and green fluorescent probes, respectively. Co-immobilization was assessed by merging the fluorescent images, which produced a yellow signal. The bioactivity of the peptide-containing surface was assessed by the adhesion, proliferation and differentiation of MC3T3-E1 murine osteoblasts.
The morphology of the MC3T3 cells, grown on various Ti surfaces, was assessed by immunofluorescence (Figure 2a-d). Cells cultured on surfaces containing the two peptides, Ti/CPTES/Mix, and cells grown on the positive-control TCPS (not shown) presented well-defined cytoskeletons and large areas with adhesion points, when compared to cells grown on untreated Ti surfaces or Ti surfaces containing only one of the two peptides. These results show that the two peptides have a synergistic effect which induces osteoblasts adhesion. The number of cells adhered to the Ti/CPTES/Mix and Ti/CPTES/RGD surfaces after two hours was significantly higher than all other surfaces, and after four hours the Ti/CPTES/Mix surface showed significantly higher results compared to all other surfaces, treated or untreated.
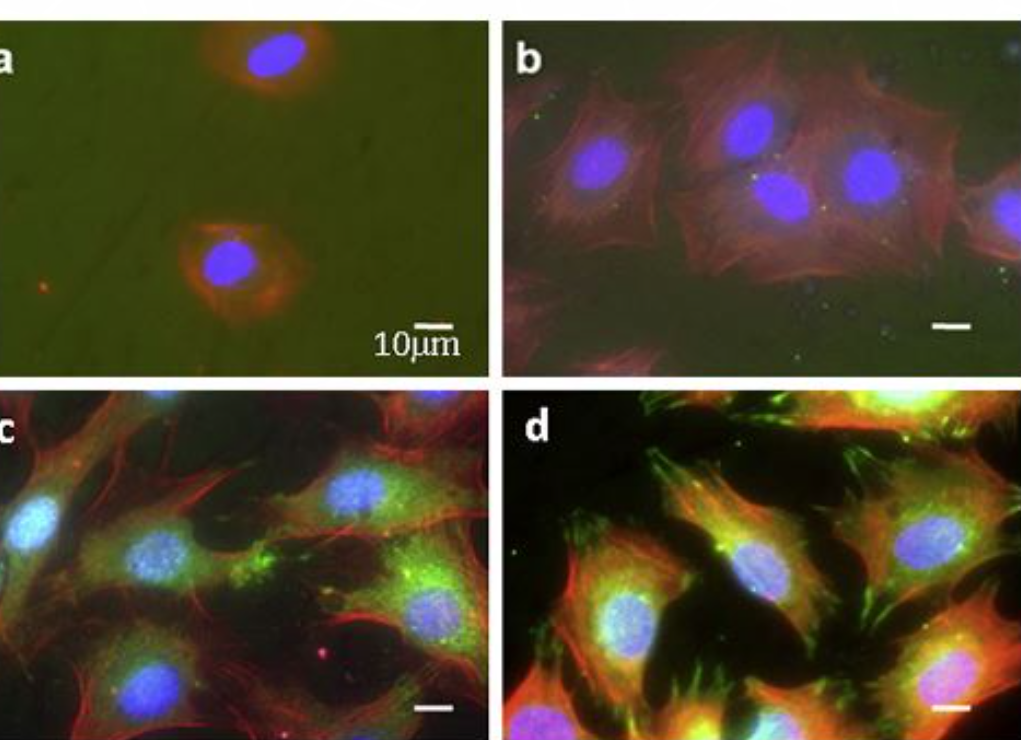
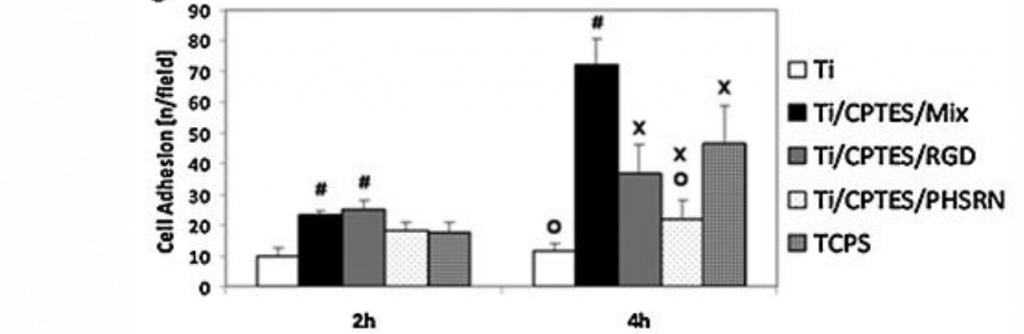
Figure 2: Adhesion of MC3T3 osteoblasts
Figure 2: Adhesion of MC3T3 osteoblasts. Fluorescent imaging of MC3T3 cells cultured on (a) untreated Ti, (b) Ti/CPTES/PHSRN, (c) Ti/CPTES/RGD and (d) Ti/CPTES/Mix. Red: actin filaments (cytoskeleton); Red: vinculin (focal adhesion points); Blue: nuclei. (e) Mean ± SD of adhered MC3T3-E1 cells on the tested surfaces after 2 and 4 h in culture. Error bars represent standard deviations of at least three samples in each group. Bars with different symbols (#, O, X) are from surfaces with statistically significant differences (p-value < 0.05) at the same time point.
The proliferation of the MC3T3 cells on the various surfaces was measured after three and five days in culture. As Figure 3 shows, the Ti/CPTES/Mix and the positive control TCPS surfaces significantly enhanced the proliferation of the osteoblasts, when compared to all other surfaces.
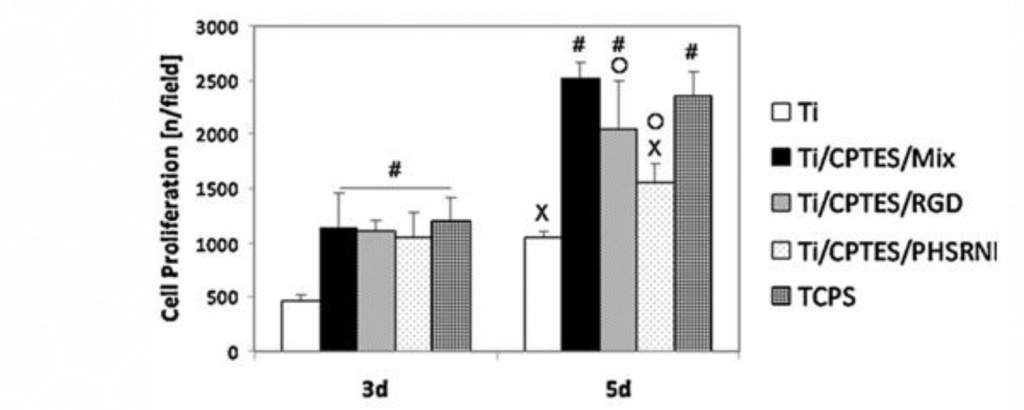
Figure 3: Proliferation of MC3T3 osteoblasts
Mean ± SD of proliferated MC3T3 cells on different surfaces after three and five days in culture. Error bars represent standard deviation of at least three samples in each group. Bars with different symbols (#, O, X) are from surfaces with statistically significant differences (p-value < 0.05) at the same time point.
Osteoblast differentiation was measured by alkaline phosphatase (ALP) activity (Figure 4), after seven and fourteen days of culture. The ALP levels measured for the Ti/CPTES/Mix were higher than all other titanium surfaces, indicating a high level of osteoblasts differentiation. These differences, however, were not statistically significant.
ALP activity (mean± SD) of MC3T3-E1 cells differentiated on different surfaces after seven and fourteen days in culture. Error bars represent the standard deviations of at least three samples in each group. Bars with different symbols (#, O, X) are from surfaces with statistically significant differences (p-value < 0.05) at the same time point.
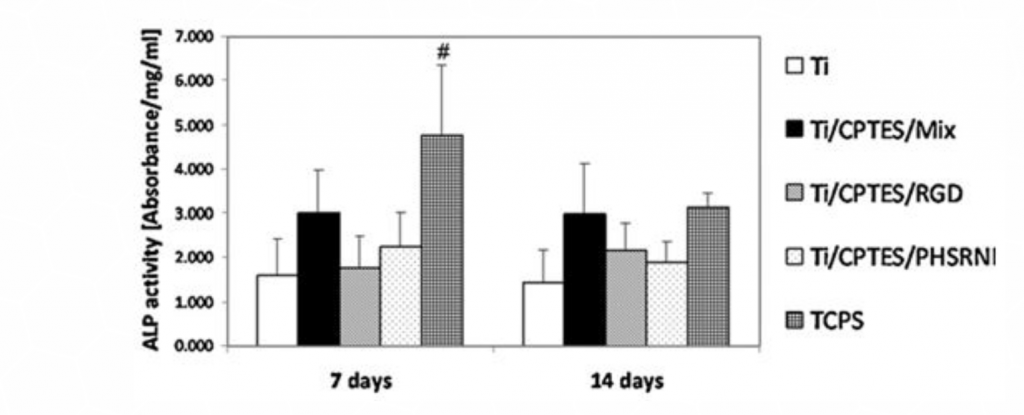
Figure 4: Differentiation of MC3T3 osteoblast
The data collected in this study shows that titanium surfaces with coatings containing the combination of motifs of two peptides, RGD and PHSRN,
were bioactive and enhanced osteoblasts proliferation, adhesion and differentiation.
This retained bioactivity is essential for the appropriate biological performance that is
required from dental and orthopedic implants.
The data collected in this study shows that titanium surfaces with coatings containing the combination of motifs of two peptides, RGD and PHSRN, were bioactive and enhanced osteoblasts proliferation, adhesion and differentiation.
This retained bioactivity is essential for the appropriate biological performance that is required from dental and orthopedic implants.
The combination of innovative surface technology with 344% stronger bone reduces marginal bone loss and provides for a higher BIC%, decreasing the risk of peri-implant disease. The enhanced
deep thread simplifies the insertion and allows
for high primary stability.
The combination of innovative surface technology with 344% stronger bone reduces marginal bone loss and provides for a higher BIC%,
decreasing the risk of peri-implant disease.
The enhanced deep thread
simplifies the insertion and
allowsfor high primary stability.
The combination of innovative surface technology with 344% stronger bone reduces marginal bone loss and provides for a higher BIC%,
decreasing the risk of peri-implant disease.
The enhanced deep thread
simplifies the insertion and
allowsfor high primary stability.
Bibliography
[1] O.E. Pohler, Unalloyed titanium for implants in bone surgery, Injury 31 (Suppl.4) (2000) 7–13.
[2] D.A. Puleo, A. Nanci, Understanding and controlling the bone–implant interface, Biomaterials 20 (1999) 2311–2321.
[3] D. Campoccia, L. Montanaro, C.R. Arciola, The significance of infection related to orthopedic devices and issues of antibiotic resistance, Biomaterials 27 (2006) 2331–2339.
[4] J.D. Bumgardner, P. Adatrow, W.O. Haggard, P.A. Norowski, Emerging antibacterial biomaterial strategies for the prevention of pen-implant inflammatory diseases, Int. J. Oral Max. Implant. 26 (2011) 553–560.
[5] R. Beutner, J. Michael, B. Schwenzer, D. Scharnweber, Biological nanofunctionalization of titanium-based biomaterial surfaces: a flexible toolbox, J R Soc Interface 7 (2010) S93–S105.
[6] H. Schliephake, D. Scharnweber, Chemical and biological functionalization of titanium for dental implants, J. Mater. Chem. 18 (2008) 2404–2414.
[7] G. Schmidmaier, B. Wildemann, F. Cromme, F. Kandziora, N.P. Haas, M. Raschke, Bone morphogenetic protein-2 coating of titanium implants increases biomechanical strength and accelerates bone remodeling in fracture treatment: a biomechanical and histological study in rats, Bone 30 (2002) 816–822.
[8] W. Xiang, L. Baolin, J. Yan, X. Yang, The effect of bone morphogenetic protein on osseointegration of titanium implants, J. Oral Maxil. Surg. 51 (1993) 647–651.
[9] S.E. Kim, S.H. Song, Y.P. Yun, B.J. Choi, I.K. Kwon, M.S. Bae, H.J. Moon, Y.D. Kwon, The effect of immobilization of heparin and bone morphogenic protein-2 (BMP-2) to titanium surfaces on inflammation and osteoblast function, Biomaterials 32 (2011) 366–373.
[10] A.T. Sverzut, G.E. Crippa, M. Morra, P.T. de Oliveira, M.M. Beloti, A.L. Rosa, Effects of type I collagen coating on titanium osseointegration: histomorphometric, cellular and molecular analyses, Biomed. Mater. 7 (2012).
[11] R. Beutner, J. Michael, A. Forster, B. Schwenzer, D. Scharnweber, Immobilization of oligonucleotides on titanium based materials by partial incorporation in anodic oxide layers, Biomaterials 30 (2009) 2774–2781.
[12] J. Michael, R. Beutner, U. Hempel, D. Scharnweber, H. Worch, B. Schwenzer, Surface modification of titanium-based alloys with bioactive molecules using electrochemically fixed nucleic acids, J. Biomed. Mater. Res. B 80B (2007) 146–155.
[13] S. Rammelt, T. Illert, S. Bierbaum, D. Scharnweber, H. Zwipp, W. Schneiders, Coating of titanium implants with collagen, RGD peptide and chondroitin sulfate, Biomaterials 27 (2006) 5561–5571.
[14] H.C. Kroese-Deutman, J. van den Dolder, P.H. Spauwen, J.A. Jansen, Influence of RGD-loaded titanium implants on bone formation in vivo, Tissue Eng. 11 (2005) 1867–1875.
[15] H.S. Seo, Y.M. Ko, J.W. Shim, Y.K. Lim, J.K. Kook, D.L. Cho, B.H. Kim, Characterization of bioactive RGD peptide immobilized onto poly(acrylic acid) thin films by plasma polymerization, Appl. Surf. Sci. 257 (2010) 596–602.
[16] S.J. Xiao, M. Textor, N.D. Spencer, M. Wieland, B. Keller, H. Sigrist, Immobilization of the cell-adhesive peptide Arg–Gly–Asp–Cys (RGDC) on titanium surfaces by covalent chemical attachment, J. Mater. Sci. Mater. M 8 (1997) 867–872.
[17] T.A. Petrie, J.E. Raynor, D.W. Dumbauld, T.T. Lee, S. Jagtap, K.L. Templeman, D.M. Collard, A.J. Garcia, Multivalent integrin-specific ligands enhance tissue healing and biomaterial integration, Sci. Transl. Med. 2 (2010).
[18] L.Z. Zhao, P.K. Chu, Y.M. Zhang, Z.F. Wu, Antibacterial coatings on titanium implants, J. Biomed. Mater. Res. B 91B (2009) 470–480.
[19] M. Lucke, G. Schmidmaier, S. Sadoni, B. Wildemann, R. Schiller, N.P. Haas, M. Raschke, Gentamicin coating of metallic implants reduces implant-related osteomyelitis in rats, Bone 32 (2003) 521–531.
[20] V. Alt, A. Bitschnau, J. Osterling, A. Sewing, C. Meyer, R. Kraus, S.A. Meissner, S. Wenisch, E. Domann, R. Schnettler, The effects of combined gentamicinhydroxyapatite coating for cementless joint prostheses on the reduction of infection rates in a rabbit infection prophylaxis model, Biomaterials 27 (2006) 4627–4634.
[21] S. Radin, J.T. Campbell, P. Ducheyne, J.M. Cuckler, Calcium phosphate ceramic coatings as carriers of vancomycin, Biomaterials 18 (1997) 777–782.
[22] L.G. Harris, S. Tosatti, M. Wieland, M. Textor, R.G. Richards, Staphylococcus aureus adhesion to titanium oxide surfaces coated with non-functionalized and peptide-functionalized poly(l-lysine)-grafted-poly(ethylene glycol) copolymers, Biomaterials 25 (2004) 4135–4148.
[23] X. Chen, Y. Li, C. Aparicio, Biofunctional coatings for dental implants, in: S. Nazarpour, M. Chaker (Eds.), Thin Films and Coatings in Biology. Biological and Medical Physics—Biomedical Engineering Series, Springer-Verlag, 2013, ISBN 978-94-007-2591-1.
[24] P.H. Chua, K.G. Neoh, E.T. Kang, W. Wang, Surface functionalization of titanium with hyaluronic acid/chitosan polyelectrolyte multilayers and RGD for promoting osteoblast functions and inhibiting bacterial adhesion, Biomaterials 29 (2008) 1412–1421.
[25] D.W. Lee, Y.P. Yun, K. Park, S.E. Kim, Gentamicin and bone morphogenic protein-2 (BMP-2)-delivering heparinized-titanium implant with enhanced antibacterial activity and osteointegration, Bone 50 (2012) 974–982.
[26] A. Nanci, J.D. Wuest, L. Peru, P. Brunet, V. Sharma, S. Zalzal, M.D. McKee, Chemical modification of titanium surfaces for covalent attachment of biological molecules, J. Biomed. Mater. Res. 40 (1998) 324–335.
[27] G. Mani, D.M. Johnson, D. Marton, V.L. Dougherty, M.D. Feldman, D. Patel, A.A. Ayon, C.M. Agrawal, Stability of self-assembled monolayers on titanium and gold, Langmuir 24 (2008) 6774–6784.
[28] J. Auernheimer, D. Zukowski, C. Dahmen, M. Kantlehner, A. Enderle, S.L. Goodman, H. Kessler, Titanium implant materials with improved riocompatibility through coating with phosphonate-anchored cyclic RGD peptides, Chembiochem 6 (2005) 2034–2040.
[29] X. Chen, P. Sevilla, C. Aparicio, Surface biofunctionalization by covalent co-immobilization of oligopeptides, Colloids and Surfaces B: Biointerfaces 107 (2013) 189– 197.
[30] M.D. Pierschbacher, E. Ruoslahti, Cell attachment activity of fibronectin can be duplicated by small synthetic fragments of the molecule, Nature 309 (1984) 30–33.
[31] S.E. Ochsenhirt, E. Kokkoli, J.B. McCarthy, M. Tirrell, Effect of RGD secondary structure and the synergy site PHSRN on cell adhesion, spreading and specific integrin engagement, Biomaterials 27 (2006) 3863–3874.
[32] D.S.W Benoit, K.S. Anseth, The effect on osteoblast function of colocalized RGD and PHSRN epitopes on PEG surfaces, Biomaterials 26 (2005) 5209–5220.
[33] W.J. Kao, D. Lee, J.C. Schense, T.A. Hubbell, Fibronectin modulates macrophage adhesion and FBGC formation: the pole of RGD, PHSRN, and PRRARV domains, J. Biomed. Mater. Res. 55 (2001) 79–88.
[34] Y.Z. Feng, M. Mrksich, The synergy peptide PHSRN and the adhesion peptide RGD mediate cell adhesion through a common mechanism, Biochemistry-US 43 (2004) 15811–15821.
[35] D.R. Schmidt, W.J. Kao, Monocyte activation in response to polyethylene glycol hydrogels grafted with RGD and PHSRN separated by interpositional spacers of various lengths, J. Biomed. Mater. Res. A 83A (2007) 617–625.
[36] T.A. Petrie, J.R. Capadona, C.D. Reyes, A.J. Garcia, Integrin specificity and enhanced cellular activities associated with surfaces presenting a recombinant fibronectin fragment compared to RGD supports, Biomaterials 27 (2006) 5459–5470.
Stay Safe. Wear a Mask!
The pink tissue versatile implant neck combines superior gingival aesthetics and high primary stability, improved placement, and temporization,
which is particularly beneficial in
aesthetically demanding cases.
The pink tissue versatile implant neck combines superior gingival aesthetics and high primary
stability, improved placement, and
temporization, which is particularly
beneficial in aesthetically demanding
cases.
The pink tissue versatile implant neck combines superior gingival aesthetics and high primary stability, improved placement, and temporization, which is particularly beneficial in aesthetically demanding cases.