OPG and RANK-L, correlation to bone formation and healing
The RANKL/OPG ratio in bone marrow is an important indication of
bone mass in both pathological and normal states.RANKL (NF-jB)
is expressed on osteoblast and stromal cell membranes and binds
to its receptor, RANK, on the surface of osteoclasts and osteoclast
precursor cells permitting the regulation of bone resorption.
Osteoprotegerin (OPG) is secreted by osteoblasts and osteogenic
stromal cells and inhibits bone resorption by binding to RANKL
and halts osteoclast activity-thus OPG protects the body from
excess bone resorption.
OPG secretion is stimulated by the sex hormone estrogen,
as well as osteoporosis drugs such as strontium ranelate and
Denosumab, proven in vivo studies. (1,2)
OPG and RANK-L, correlation to bone formation and healing
The RANKL/OPG ratio in bone marrow is an
important indication of bone mass in both pathological and
normal states.RANKL (NF-jB) is expressed on osteoblast
and stromal cell membranes and binds to its receptor,
RANK, on the surface of osteoclasts and osteoclast
precursor cells permitting the regulation
of bone resorption.
Osteoprotegerin (OPG) is secreted by
osteoblasts and osteogenic stromal cells
and inhibits bone resorption by binding
to RANKL and halts osteoclast activity-thus
OPG protects the body from excess bone
resorption.
OPG secretion is stimulated by the sex hormone
estrogen, as well as osteoporosis drugs such as
strontium ranelate and Denosumab, proven
in vivo studies. (1,2)
OPG and RANK-L, correlation to bone formation and healing
The RANKL/OPG ratio in bone marrow is an important indication of
bone mass in both pathological and normal states.RANKL (NF-jB)
is expressed on osteoblast and stromal cell membranes and binds
to its receptor, RANK, on the surface of osteoclasts and osteoclast
precursor cells permitting the regulation of bone resorption.
Osteoprotegerin (OPG) is secreted by osteoblasts and osteogenic
stromal cells and inhibits bone resorption by binding to RANKL
and halts osteoclast activity-thus OPG protects the body from
excess bone resorption.
OPG secretion is stimulated by the sex hormone estrogen,
as well as osteoporosis drugs such as strontium ranelate and
Denosumab, proven in vivo studies. (1,2)
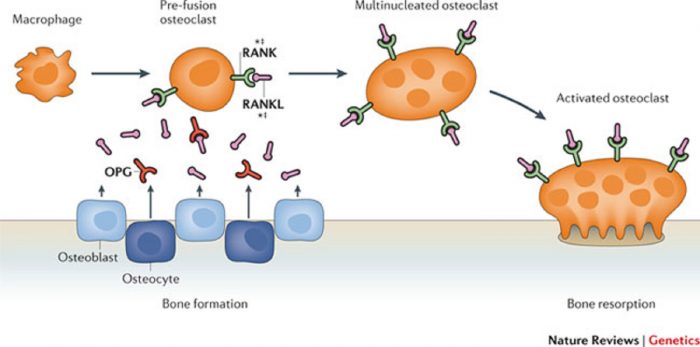
Osteoclast differentiation requires RANKL and RANK expression on osteoblastic stromal cells and osteoclast precursor cells respectively.
Many of the factors that were found to induce RANKL transcription were
found to also induce the transcription of OPG.
However, the direct correlation of the two factors is counterintuitive to their inverse regulation: when there is upregulation of RANKL there is downregulation of OPG.
This finding suggests that the induction of upregulation of OPG will decrease the production of RANKL, such that the RANKL/OPG ratio moves in favor of osteoblast differentiation. (5)
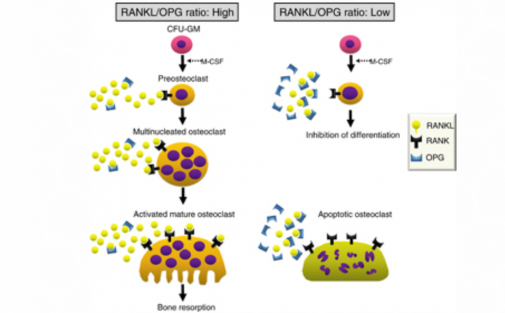
Osteoclast differentiation requires RANKL and RANK expression on osteoblastic stromal cells and osteoclast precursor cells respectively.
Many of the factors that were found to induce RANKL transcription were
found to also induce the transcription of OPG.
However, the direct correlation of the two factors is counterintuitive to their inverse regulation: when there is upregulation of RANKL there is downregulation of OPG.
This finding suggests that the induction of upregulation of OPG will decrease the production of RANKL, such that the RANKL/OPG ratio moves in favor of osteoblast differentiation. (5)
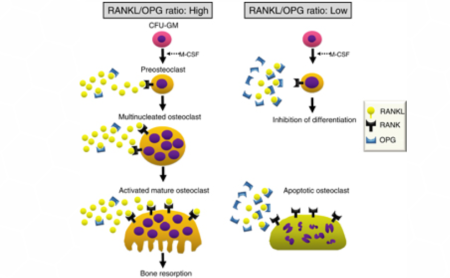
It should be noted that when comparing mice with and without OPG transcription (based on mutated genes i.e. OPG/apoE double knockout) it was found that the mice lacking OPG had a significant acceleration of calcification of atherosclerotic plaques. This suggests that OPG also plays a role in the calcification processes in atherosclerosis. (5)
A direct correlation between OPG levels and the micro-roughness of titanium implants was found in both in vivo and in vitro studies.
A 350% increase in OPG levels was found when the titanium implant was treated with 1α, 25(OH)2D3(osteogenesis stimulating factor) with a
the roughness of 5 μm as opposed to 50% on a treated surface
with a roughness of less than 0.2 μm.
The rise in OPG was coupled with increased differentiation of osteoblasts indicating higher mineral bone density and bone volume in surfaces with increased micro-roughness. (3)
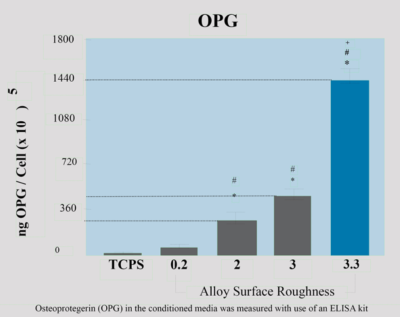
In another study, there was a correlation between rough micro-topography and increased OPG levels without the treatment of osteogenesis-stimulating factors such as 1α,25(OH)2D3. Rough micro-topography in and of itself causes increased secretion of stimulating factors (including estradiol-17 and 1α,25(OH)2D3) which allows the researcher to measure OPG levels without the intervention of other factors. The study suggests that the cause of increased OPG levels is due to increased OPG mRNA. In contrast, RANKL mRNA levels are very low and no soluble RANKL is seen in rough surface implants. (4)
DENTALIS surface causes a significant increase in OPG concentration, increases the level of differentiated osteoblasts and improves bone formation and healing.

References:
- Boyce, Brendan F., and Lianping Xing. “The RANKL/RANK/OPG pathway.” Current osteoporosis reports 5.3 (2007): 98-104. Khosla S (December 2001). “Minireview: the OPG/RANKL/RANK system”. Endocrinology. 142 (12): 5050–5. doi:10.1210/en.142.12.5050. PMID 11713196.
- Lossdörfer, S., Schwartz, Z., Wang, L., Lohmann, C. H., Turner, J. D., Wieland, M., Cochran, D. L. and Boyan, B. D. (2004), Micro rough implant surface topographies increase osteogenesis by reducing osteoclast formation and activity. J. Biomed. Mater. Res., 70A: 361–369. doi:10.1002/jbm.a.30025
- Boyan, B. D., et al. “Osteoblasts generate an osteogenic microenvironment when grown on surfaces with rough microtopographies.” Eur Cell Mater 6.24 (2003): 22-27.
- Costa-Rodrigues, João, et al. “Hydroxyapatite surface roughness: complex modulation of the osteoclastogenesis of human precursor cells.” Acta biomaterials 8.3 (2012): 1137-1145.
- Boyce, Brendan F., and Lianping Xing. “Functions of RANKL/RANK/OPG in Bone Modeling and Remodeling.” Archives of biochemistry and biophysics473.2 (2008): 139–146. PMC. Web. 3 Dec. 2016.
- Kajiya, Mikihito, Gabriela Giro, Martin A. Taubman, Xiaozhe Han, Marcia P.A. Mayer, and Toshihisa Kawai. “Role of Periodontal Pathogenic Bacteria in RANKL-mediated Bone Destruction in Periodontal Disease.” Role of Periodontal Pathogenic Bacteria in RANKL-mediated Bone Destruction in Periodontal Disease | Kajiya | Journal of Oral Microbiology. Journal of Oral Microbiology 2010, 2: 5532 – DOI: 10.3402 / jom.v2i0.5532, 8 Nov. 2010. Web. 03 Dec. 2016.
- Richards, J. Brent, Hou-Feng Zheng, and Tim D. Spector. “Genetics of osteoporosis from genome-wide association studies: advances and challenges.” Nature Reviews Genetics 13.8 (2012): 576-588.
The combination of innovative surface technology with 344% stronger bone reduces marginal bone loss and provides for a higher BIC%, decreasing the risk of peri-implant disease.
The combination of innovative surface technology with 344% stronger bone reduces marginal bone loss and provides for a higher BIC%, decreasing the risk of peri-implant disease.