The effect of micro-topography of the implant surface on osteoblasts and bone formation
The surface of the bone and dental implants plays an important role in osteoblast adhesion and bone growth. Many parameters affect the quality of osteointegration, including chemical composition and surface morphology [1]. The microtopography of the surface appears to be an important factor in the determination of the level of biological interaction between tissues and biomaterials, and many methods are used to achieve surface roughness on the micro-and-nano scale [2]. In-vivo studies have shown that, while smooth surfaces encourage the formation of fibrous tissue, surfaces with micrometer-scale roughness are more conducive to bone formation [3-5]. These studies established that certain surfaces regulate osteoblast adhesion, proliferation, mineralization, and differentiation [6-9], and when osteoblasts interact with implant surfaces with micro-scale topography they differentiate to mature osteoblasts, producing increased levels of alkaline phosphatase, osteocalcin [1], growth factors, cytokines, and prostaglandins, all of which promote osteoblastogenesis and inhibit osteoclast activity [10,11]. In addition, these surfaces exhibit improved contact with bone [12-14], provide more mechanical support and encourage osteointegration more quickly compared to implants with smooth surfaces [5,15-17].
Figure 1 [18] shows the surface morphology of four different Ti6Al4V discs: one is smoothed by a machine with an average roughness (Ra) of 0.2 µm, and the others are grit-blasted with Ra of 2.0, 3.0, and 3.3 µm. While the smoothed disc exhibits a concentric pattern due to the smoothing process, the grit-blasted surfaces are uneven, containing grooves and cracks.

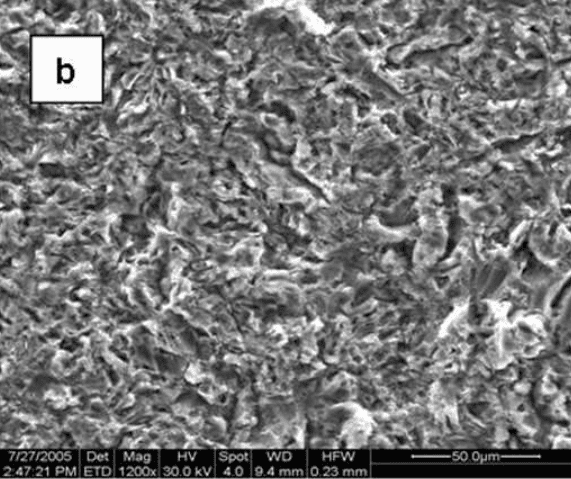

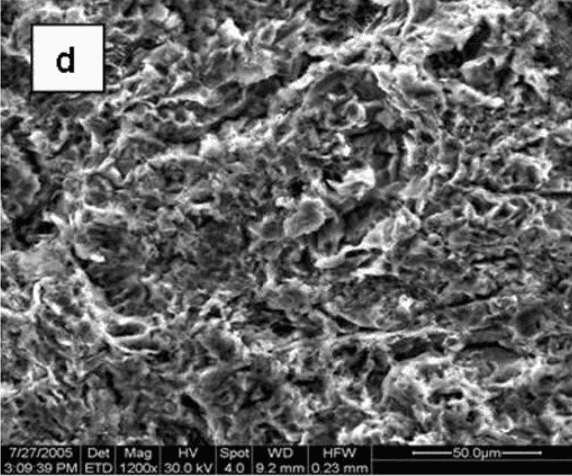
Fig. 1: Scanning electron micrographs showing the morphology of the surfaces of the Ti6Al4V substrates: machine-polished (a), grit-blasted with Ra values of 2.0, 3.0, and 3.3 µm (b, c, and d, respectively) (X1200).
Cell morphology
Cellular morphology was sensitive to the disk surface properties. When osteoblast-like MG63 cells were grown on TCPS (data not shown) or smooth Ti alloy surfaces, the cells were spindle shape and elongated. In addition, they were aligned with the microgroove structure of the machined disks (Figure 2). On the rougher, grit-blasted surfaces, most cells appeared triangular, polygonal or rounded with cytoplasmic extensions into the pits of the blasted topography.

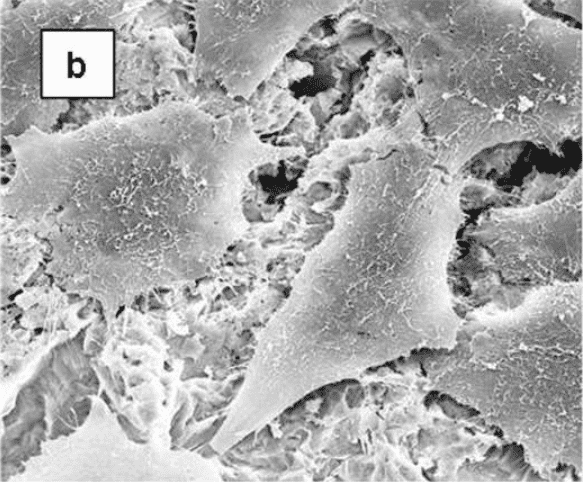
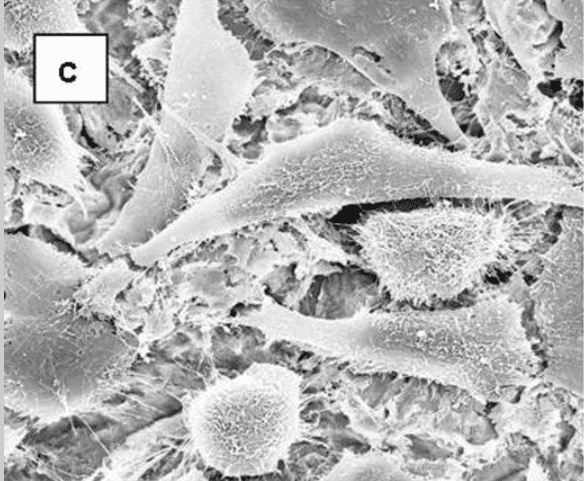
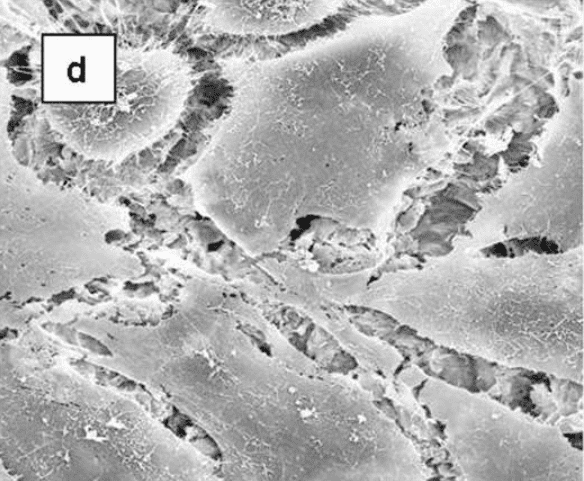
Scanning electron micrographs showing the morphology of MG63 cells on microstructured Ti6Al4V substrates. MG63 cells were cultured on machined (a) or grit-blasted Ti6Al4V substrates with Ra of 2.0, 3.0, or 3.3 μm (b-d, respectively) for 6 days. The cell morphologies were observed by SEM (x 1,000).
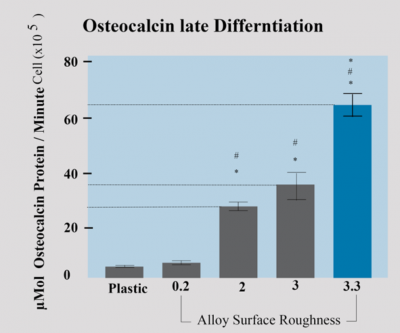
The micro-topography of the implant surface also has an effect on the differentiation of the osteoblasts associated with the surface, as well as on the production of local factors. The figure shows the quantification of various factors secreted from the osteoblasts that underwent adhesion to the same surfaces shown in Figure 1. The number of cells and amount of alkaline phosphatase (an early marker of osteoblast differentiation) were reduced on Ti6Al4V surfaces (compared to tissue-culture polystyrene), and both parameters were lower on surfaces with rough topography compared to a smooth surface (data not shown). However, the amount of osteocalcin, a late differentiation marker of osteoblasts, both in the condition media (Fig. 2-a) and normalized per cell (Fig. 6-b), was higher on Ti6Al4V surfaces compared to TCPS, and increased significantly on roughened surfaces than on smooth surfaces. These data suggest that despite the lower number of cells on surfaces with micro-topography, the osteoblasts attached to them are more differentiated and secrete more local factors.

In-vitro research on DENTALIS surface with 3.3µ provided favorable results:
In-vitro research on DENTALIS surface with 3.3µ provided favorable results:
Osteocalcin is a late marker of differentiation and increases as the mineral is deposited. Osteoblasts were cultured on the Dentalis roughing surface with 3.3µ displayed.
The osteocalcin levels increased 11-fold on rough surfaces of 3.3µ compared to smooth surfaces.
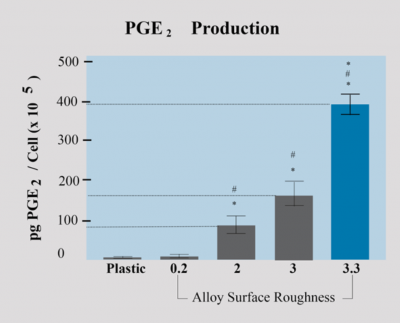
Growth factors PGE2 - Prostaglandin
It is required for osteoblast activity and prostaglandins mediate cell response to the surface structure. Studies show that levels of local growth factors like PGE2-prostaglandin are significantly increased when osteoblasts are cultured on the Dentalis BAS surface, as compared to plastic or pre-treated titanium disks.
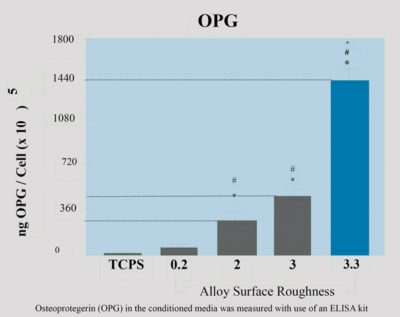
The RANKL-OPG system in the osteoblast osteoclast coupling
Physiologic and reactive bone remodeling relies on tight communication (coupling) between osteoblasts and osteoclasts. A critical component of this communication depends on the RANKL OPG system. Both membrane-bound and excreted RANKL (produced by the osteoblasts) mediate a signal for osteoclast formation through RANK (“RANKL receptor”) expressed on osteoclast progenitors. OPG (also produced and excreted by the osteoblasts) counteracts this effect by competing for and neutralizing RANKL. Tightly controlled bone remodeling is critical for the adaptation of bone to mechanical stresses and bone repair, including implant osseointegration.
Dentalis surface will provide a higher level of OPG & RANKL
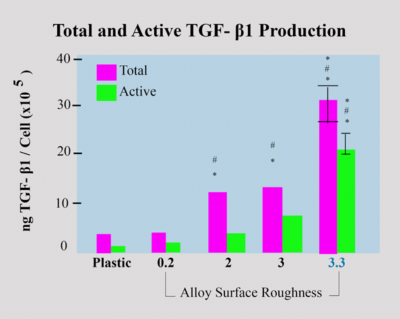
TGF- β1 Production Local factor production
These growth factors stimulate matrix synthesis and differentiation of osteoblasts and also inhibit osteoclasts. Another major difference can be observed in TGF-β levels. Dentalis BAS surface leads to a ten-fold increase in TGF-β production, compared to the control group.
Effects of surface roughness on osteoblast differentiation and local factor production. MG63 cells were grown on tissue culture polystyrene, Ti6AVI4V surfaces with the smoothed surface, and various roughnesses (2.0 µm, 3.0 µm, and 3.3 µm)while the 3.3µm provide the higher level.
This resulted as well in a significant difference in removal torque measurements between the smooth and Dentalis rough-surfaced screws.
Smooth screws required 2.3 ± 0.3 Nm, while Dentalis rough screws required 5.3 ± 0.4 Nm
In-vivo experiments also confirmed the advantage of micro-topography of the implant surface in terms of osteoblast growth, adhesion, and mineralized matrix production. Although some bone was formed on the smooth surface implants, it was also covered with fibrous tissue (Fig. 3-a,b,c), which was detached from the surface during the histological processing.
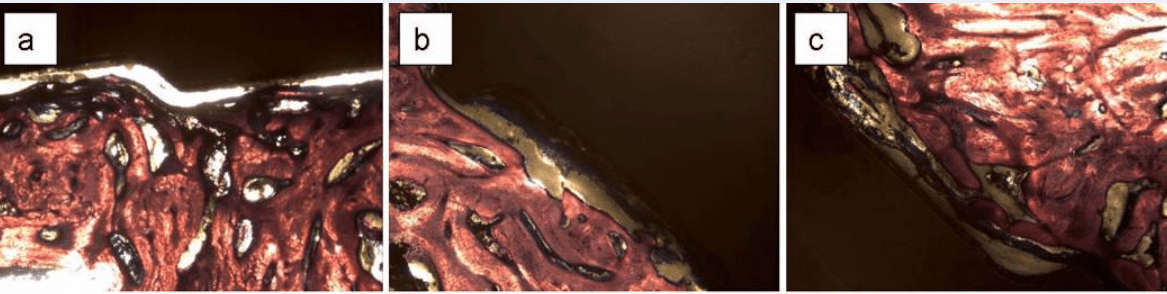
Some fibrous tissue was found also on roughened surfaces (Fig. 3-d,e,f), but it was thinner and more attached to the surface so the detachment was mostly within the bone rather than at the bone-implant interface. Bone cells were directly attached to the rough surfaces and produced a mineralized matrix.
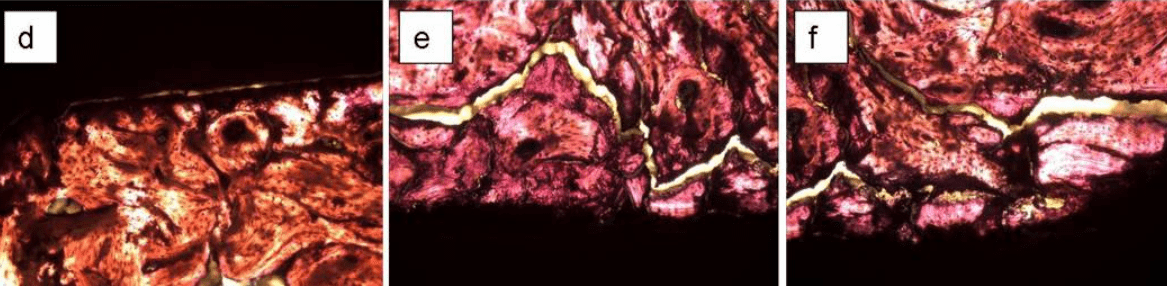
Fig. 3: histological sections oh bone formed on Ti6Al4V pedicle screws implanted in the L4 vertebra of sheep. The screws had smooth (a,b,c) while (c,d,e) rough surfaces with Dentalis 3.3µm

For more info about BAS ™ Surface and the Scientific Research
Documentation In vitro & In vivo experiments please contact your local distributor.
The combination of innovative surface technology
with 344% stronger bone reduces marginal
bone loss and provides for a higher BIC%,
decreasing the risk of peri-implant disease.
The enhanced deep thread
simplifies the insertion and
allows for high
primary stability.
The combination of innovative surface technology with 344% stronger bone reduces marginal bone loss and provides for a higher BIC%, decreasing the risk of peri-implant disease. The enhanced
deep thread simplifies the insertion and allows
for high primary stability.
The combination of innovative surface technology with 344% stronger bone reduces marginal bone loss and provides for a higher BIC%, decreasing the risk of peri-implant disease. The enhanced
deep thread simplifies the insertion and allows
for high primary stability.
Abbreviations:
- 1a,25 (OH)2D3 1a,25-Dihydroxy vitamin D3
- Ang-1 Angiopoietin 1
- BIC Bone to implant contact
- BMP-2 Bone morphogenetic protein 2
- Cox Cyclooxygenase
- CSR Cumulative success rate
- DAG Diacylglycerol
- EGF Epidermal growth factor
- ERK1/2 Extracellular regulated kinase
- FGF-2 Fibroblast growth factor 2 (basic fibroblast growth factor)
- GFOGER Glycine-phenylalanine-hydroxyprolineglycine-glutamate-arginine
- KRSR Lysine-arginine-serine-arginine
- KSSR Lysine-serine-serine-arginine
- LPA Lysophosphatidic acid
- MAPK Mitogen-activated protein kinase
- MSC Mesenchymal stem cell
- OPG Osteoprotegerin
- PA Phosphatidic acid
- PC Phosphatidylcholine
- PGE1 Prostaglandin E1
- PGE2 Prostaglandin E2
- PKC Protein kinase C
- PLD Phospholipase D
- RANK Receptor activator of nuclear factor kB
- RANKL Receptor activator of nuclear factor kB ligand
- RGD Arginine-glycine-aspartic acid
- TGF-b1 Transforming growth factor b1
- VEGF-A Vascular endothelial growth factor A
Bibliography
1. Boyan BD, Lossdörfer S, Wang L, Zhao G, Lohmann CH, Cochran DL, Schwartz Z. Osteoblasts generate an osteogenic microenvironment when grown on surfaces with rough microtopographies. Eur Cell Mater. 2003;6:22-7.
2. Bagno A, Di Bello C. Surface treatments and roughness properties of Ti-based biomaterials. J Mater Sci Mater Med. 2004;15:935-49.
3. Brunette DM. Spreading and orientation of epithelial cells on grooved substrata. Exp Cell Res. 1986;167:203-17.
4. Thomas KA, Cook SD. An evaluation of variables influencing implant fixation by direct bone apposition. J Biomed Mater Res. 1985;19:875-901.
5. Cochran DL. A comparison of endosseous dental implant surfaces. J Periodontol. 1999;70:1523-39.
6. Martin JY, Schwartz Z, Hummert TW, Schraub DM, Simpson J, Lankford J Jr, Dean DD, Cochran DL, Boyan BD. Effect of titanium surface roughness on proliferation, differentiation, and protein synthesis of human osteoblast-like cells (MG63). J Biomed Mater Res. 1995;29:389-401.
7. Brett PM, Harle J, Salih V, Mihoc R, Olsen I, Jones FH, Tonetti M. Roughness response genes in osteoblasts. Bone. 2004;35:124-33.
8. Guizzardi S, Galli C, Martini D, Belletti S, Tinti A, Raspanti M, Taddei P, Ruggeri A, Scandroglio R. Different titanium surface treatment influences human mandibular osteoblast response. J Periodontol. 2004;75:273-82.
9. Keller JC, Schneider GB, Stanford CM, Kellogg B. Effects of implant microtopography on osteoblast cell attachment. Implant Dent. 2003;12:175-81.
10. Ong JL, Carnes DL, Cardenas HL, Cavin R. Surface roughness of titanium on bone morphogenetic protein-2 treated osteoblast cells in vitro. Implant Dent. 1997;6:19-24.
11. Boyan BD, Batzer R, Kieswetter K, Liu Y, Cochran DL, Szmuckler-Moncler S, Dean DD, Schwartz Z. Titanium surface roughness alters the responsiveness of MG63 osteoblast-like cells to 1 alpha,25-(OH)2D3. J Biomed Mater Res. 1998;39:77-85.
12. Cochran DL, Schenk RK, Lussi A, Higginbottom FL, Buser D. Bone response to unloaded and loaded titanium implants with a sandblasted and acid-etched surface: a histometric study in the canine mandible. J Biomed Mater Res. 1998;40:1-11.
13. Gotfredsen K, Wennerberg A, Johansson C, Skovgaard LT, Hjørting-Hansen E. Anchorage of TiO2-blasted, HA-coated, and machined implants: an experimental study with rabbits. J Biomed Mater Res. 1995;29:1223-31.
14. Buser D, Schenk RK, Steinemann S, Fiorellini JP, Fox CH, Stich H. Influence of surface characteristics on bone integration of titanium implants. A histomorphometric study in miniature pigs. J Biomed Mater Res. 1991;25:889-902.
15. Khang W, Feldman S, Hawley CE, Gunsolley J. A multi-center study comparing dual acid-etched and machined-surfaced implants in various bone qualities. J Periodontol. 2001;72:1384-90.
16. Albrektsson T, Wennerberg A. Oral implant surfaces: part 2—review focusing on clinical knowledge of different surfaces. Int J Prosthodont. 2004;17:544-64.
17. Buser D, Broggini N, Wieland M, Schenk RK, Denzer AJ, Cochran DL, Hoffmann B, Lussi A, Steinemann SG. Enhanced bone apposition to a chemically modified SLA titanium surface. J Dent Res. 2004;83:529-33.
18. Schwartz Z, Raz P, Zhao G, Barak Y, Tauber M, Yao H, Boyan B. Effect of micrometer-scale roughness of the surface of Ti6Al4V pedicle screws in vitro and in vivo. J Bone Joint Surg Am. 2008;90:2485-98.
The pink tissue versatile implant neck combines superior gingival aesthetics and high primary stability, improved placement,
and temporization, which is particularly beneficial in
aesthetically demanding cases.
REFERENCES:
1.Bittner N, Schulze-Späte U, Cleber S, Da Silva J, Kim D, Tarnow D, Ishikawa-Nagai S, Gil M. Comparison of Peri-implant Soft Tissue Color with the Use of Pink-Neck vs Gray Implants and Abutments Based on Soft Tissue Thickness:
A 6-Month Follow-up Study. Int J Prosthodont. 2020Jan/Feb;33(1):29-38.
2. Gil M, Ishikawa-Nagai S, Elani H, Da Silva J, Kim D, Tarnow D, Schulze-Späte U, Cleber S, Bittner N. Comparison of the Color Appearance of Peri-implant Soft Tissue with Natural Gingiva Using Anodized Pink-Neck Implants and Pink Abutments: A Prospective Clinical Trial. Int J Oral Maxillofac Implants. 2019 May/June;34(3):752–758.
3.Gil M, Ishikawa-Nagai S, Elani H. A prospective clinical trial to assess the optical efficacy of pink neck implants and pink abutments on soft tissue aesthetics. J Esthet Restor Dent. 2017;29(6):1-7.38.
The pink tissue versatile implant neck combines superior gingival aesthetics and high primary stability, improved placement, and temporization, which is particularly beneficial in aesthetically demanding cases.

REFERENCES:
1.Bittner N, Schulze-Späte U, Cleber S, Da Silva J, Kim D, Tarnow D, Ishikawa-Nagai S, Gil M. Comparison of Peri-implant Soft Tissue Color with the Use of Pink-Neck vs Gray Implants and Abutments Based on Soft Tissue Thickness:
A 6-Month Follow-up Study. Int J Prosthodont. 2020Jan/Feb;33(1):29-38.
2. Gil M, Ishikawa-Nagai S, Elani H, Da Silva J, Kim D, Tarnow D, Schulze-Späte U, Cleber S, Bittner N. Comparison of the Color Appearance of Peri-implant Soft Tissue with Natural Gingiva Using Anodized Pink-Neck Implants and Pink Abutments: A Prospective Clinical Trial. Int J Oral Maxillofac Implants. 2019 May/June;34(3):752–758.
3.Gil M, Ishikawa-Nagai S, Elani H. A prospective clinical trial to assess the optical efficacy of pink neck implants and pink abutments on soft tissue aesthetics. J Esthet Restor Dent. 2017;29(6):1-7.38.