The challenges of
peri-implantitis
The challenges of
peri-implantitis
Patients who are either partially or completely edentulous now have more treatment options due to dental implant-supported restorations. A dental implant is an artificial tooth root that is placed directly into the jaw and supports a prosthetic crown, replacing the tooth functionally and esthetically. It is widely considered a predictable and reliable treatment modality. However, it has become clear that even though this popular treatment is effective in the majority of patients, implants do occasionally experience issues and failures, requiring substantial periodontal and prosthodontic maintenance over time (1, 2). These difficulties primarily refer to inflammation brought on by a bacterial challenge. Peri-implant mucositis and peri-implantitis (PI) are the two clinical variations that can be identified. While both disorders share the presence of an inflammatory lesion, only the later variety manifests with loss of supporting bone, indicating that the accumulation of a bacterial biofilm causes an inflammatory state of the soft tissue and bone, leading to bone atrophy (3, 4).
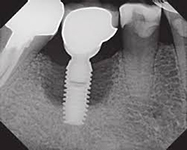
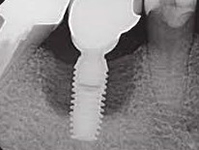
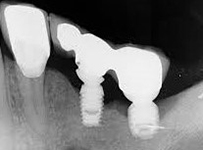
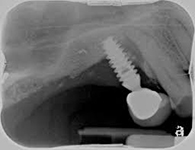
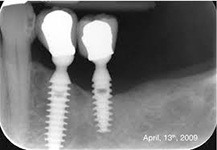
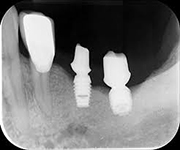
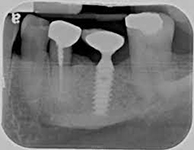
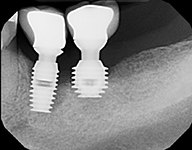
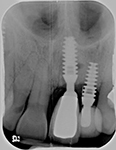
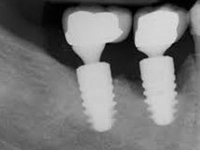
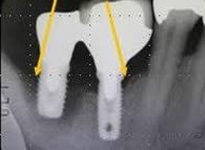
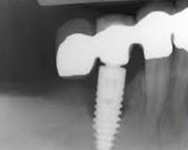
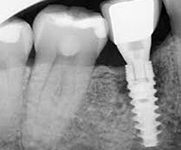
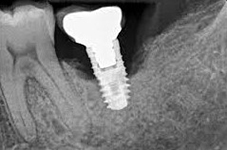
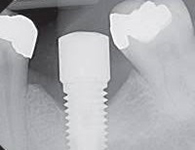
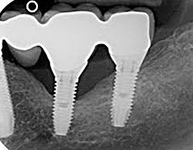
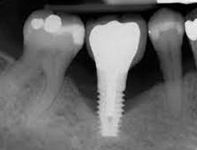
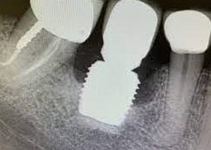
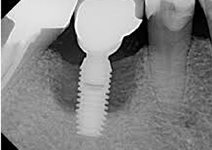

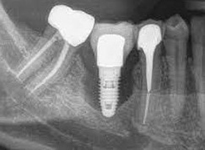
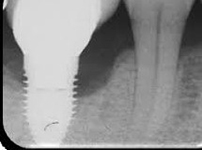

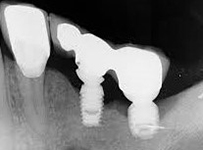
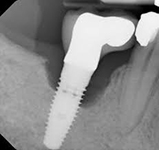
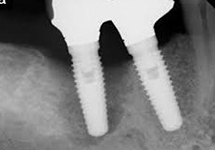
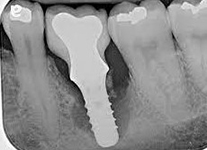
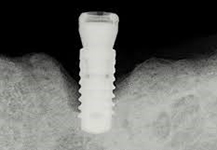
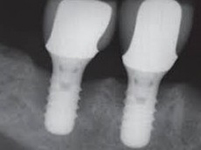
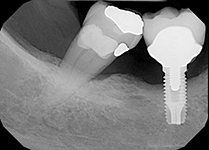
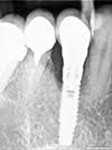
Despite this vast knowledge surrounding PI, the incidence of PI is still very common, ranging from a prevalence of 28-51%. In a study examining the incidence of PI conducted by Derks et. al, PI was found in 45% of all patients. 14.5% of cases were found to have moderate or severe peri-implantitis. Patients with periodontitis and less than four implants, as well as those who had prosthetic therapy from general practitioners and implants of particular brands, showed greater odds ratios for moderate-to-severe PI (13).
While there is no consistent treatment available for PI, multiple material solutions to prevent PI have been developed by Dentalis®.
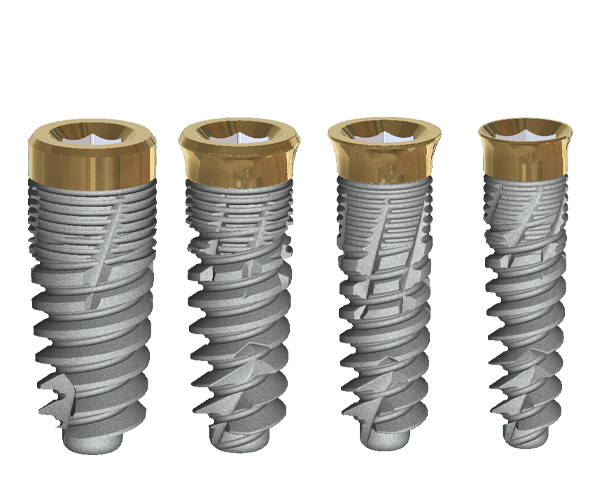
The tissue-level implant minimizes the size of the micro-gap where bacteria can colonize, and promotes stability, leading to better wound healing. These implants have been shown to minimize the occurrence of PI and are a viable alternative solution to bone-level implants (13, 14).
The second major preventative measure is an anti-bacterial coating on the implants. The coating offered by Dentalis® has been shown to minimize bacterial adhesion and promote osteoblast activity, meaning that the coating stimulates osseointegration and can prevent PI (15) .
Dentalis® products offer various preventative options for PI to maximize the quality, longevity, comfort, and esthetic results for patients requiring dental implant restorations.
References
- F. Schwarz, J. Derks, A. Monje, H. L. Wang, Peri‐implantitis. Journal of clinical periodontology 45, S246-S266 (2018).
- V. Wilson, An insight into peri-implantitis: a systematic literature review. Prim Dent J 2, 69-73 (2013).
- N. U. Zitzmann, T. Berglundh, Definition and prevalence of peri-implant diseases. J Clin Periodontol 35, 286-291 (2008).
- H. Algraffee, F. Borumandi, L. Cascarini, Peri-implantitis. British Journal of Oral and Maxillofacial Surgery 50, 689-694 (2012).
- S. Renvert, G. R. Persson, F. Q. Pirih, P. M. Camargo, Peri‐implant health, peri‐implant mucositis, and peri‐implantitis: Case definitions and diagnostic considerations. Journal of clinical periodontology 45, S278-S285 (2018).
- P. Sahrmann et al., The microbiome of peri-implantitis: a systematic review and meta-analysis. Microorganisms 8, 661 (2020).
- G. P. Mardegan et al., Transforming growth factor‐β, interleukin‐17, and IL‐23 gene expression profiles associated with human peri‐implantitis. Clinical Oral Implants Research 28, e10-e15 (2017).
- D. Lauritano et al., The impact of implant–abutment connection on clinical outcomes and microbial colonization: A narrative review. Materials 13, 1131 (2020).
- S. Raikar et al., Factors affecting the survival rate of dental implants: A retrospective study. Journal of International Society of Preventive & Community Dentistry 7, 351 (2017).
- S. Renvert, A. Aghazadeh, H. Hallstrom, G. R. Persson, Factors related to peri-implantitis – a retrospective study. Clin Oral Implants Res 25, 522-529 (2014).
- S. Ohba et al., Assessment of safety and efficacy of antimicrobial photodynamic therapy for peri-implant disease. Photodiagnosis and Photodynamic Therapy 31, 101936 (2020).
- D. Rokaya, V. Srimaneepong, W. Wisitrasameewon, M. Humagain, P. Thunyakitpisal, Peri-implantitis update: Risk indicators, diagnosis, and treatment. European journal of dentistry 14, 672-682 (2020).
- J. Derks et al., Effectiveness of implant therapy analyzed in a Swedish population: prevalence of peri-implantitis. Journal of dental research 95, 43-49 (2016).
- L. Canullo, M. Menini, F. Bagnasco, N. Di Tullio, P. Pesce, Tissue-level versus bone-level single implants in the anterior area rehabilitated with feather-edge crowns on conical implant abutments: An up to 5-year retrospective study. The Journal of Prosthetic Dentistry 128, 936-941 (2022).
- S. E. Camargo et al., Novel coatings to minimize bacterial adhesion and promote osteoblast activity for titanium implants. Journal of Functional Biomaterials 11, 42 (2020).
