The effect of titanium-implant surfaces on the behavior and characteristics of osteoblasts
The effect of titanium-implant surfaces on the behavior and characteristics of osteoblasts
One of the strongest predictors of the clinical success of orthopedic and dental implants is the osteointegration of the implant to the damaged bone [1,2], a factor influenced also by the chemistry and topography of the surface of the implant [3-5]. These characteristics are critical for the development of appropriate bone formation in the healing area surrounding the implant.
After implantation, the surface of the implant is in contact with body fluids and tissues and thereby interacts with various cells, the most important of which are osteoblasts. Osteoblasts produce the extracellular matrix and assist in building bone, and are a major consideration in implant design; one of the main challenges in designing bone implants is efficiently attracting and activating osteoblasts. To produce mineralized bone, the osteoblasts must first adhere to the surface. Studies have shown that the adhesion, proliferation, and differentiation of osteoblasts are related to the energy and roughness of the surface [6,7]; however, despite the vast amount of research concerning the field of implant surface roughness and its influence on osteoblasts, the optimal surface properties for the ideal adhesion and activation of osteoblasts have yet to be determined. At this time, most oral implants have moderate surface roughness, between 1.0 to 2.0 µm [3]. Rough surfaces encourage the entrapment of proteins, such as fibrin, and improve the mechanical properties of the implants [8-10].
Roughened implant surfaces can be created via several methods, most commonly by aluminum or titanium oxide grit-blasting followed by acid-etching [11]. This method, however, may be problematic because it may lead to contamination of the surface by aluminum or titanium particles, which can result in inflammatory reactions and impaired bone formation [12,13]. Another method roughens the surfaces of titanium implants by using biocompatible and absorbable blasting materials, for example, biphasic calcium phosphate (BCP) ceramic particles [14]. Calcium-phosphate-based materials are sensitive to acid, making them easy to remove after the blasting process [15,16]. Moreover, animal experiments examining torque force and bone formation demonstrated a positive influence of BCP grid-blasting of implant surfaces, which can be attributed to the nano-scale topography created by this blasting method, which seems to increase the absorption of proteins and adhesion of osteoblasts [17-20]. This method results in random topography and a variety of chemical compositions, so the optimal properties of titanium implant surfaces are yet to be established [21].

In 2007, Le Guehennec et. al [22] compared the behavior of osteoblasts on various titanium surfaces. Four different groups were investigated: mirror-polished (Smooth-Ti), alumina-blasted and acid-etched (Alumina-Ti), sand-blasted, large-grit, acid-etched (SLA), and biphasic calcium phosphate grit-blasted and acid-etched (BCP-Ti). Figure 1 [22] displays the surfaces of the 4 investigated implants. Smooth-Ti shows parallel slits that are the result of the manufacturing process (Fig. 1-A), while the other three grid-blasted surfaces display a rougher topography. In addition, residual alumina particles can be observed, embedded in the surface. The SLA implant showed a complex surface topography (Fig. 1-C), and the BCP-Ti surface presented irregular surface morphology.
No residual particles could be found on the BCP-Ti implant after the acid-etching and cleaning, and it also had the roughest surface (data not shown).
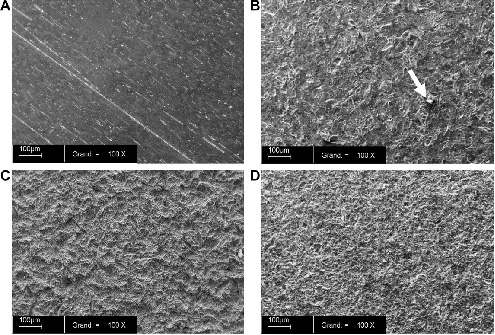
Figure 1: Scanning electron microscope (SEM) analysis of the surfaces of the four implants: A – Smooth-Ti, B – Alumina-Ti, C – SLA, D – BCP-Ti. The white arrow in B points to a residual alumina particle. Magnification X 100. Bar = 100 µm.
Table 1 [22] shows the quantification of different elements that were left embedded on the surface of the implants after all the stages of processing.
Table 1: Semi-quantitative XPS analysis of the surfaces of the 4 different implant types.
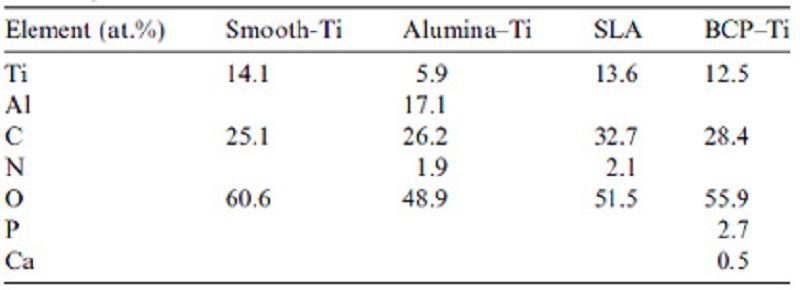
Figure 2: SEM micrographs of the MC3T3-E1 cells grown on different surfaces for 2 days. A – Smooth-Ti, B – Alumina-Ti, C – SLA, and D – BCP-Ti. Magnification X 1000. Bar = 10µm.
All surfaces were covered with a uniform layer of osteoblasts, even though they spread less on the rough titanium surfaces compared with the polished surface. Cells grown on the SLA surfaces developed more cytoplasmic extensions compared to Alumina-Ti and BCP-Ti.
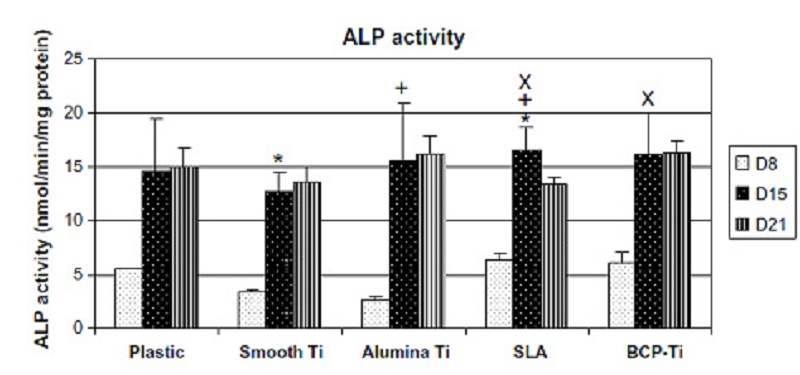
The differentiation of the MC3T3-E1 cells grown on the different titanium surfaces and plastic surfaces (control) was assessed by alkaline phosphatase (ALP) activity assay (Figure 3) [22], ALP being an early marker for osteoblasts differentiation.
ALP activity increased with time, as expected, and reached its highest levels after 15 days.
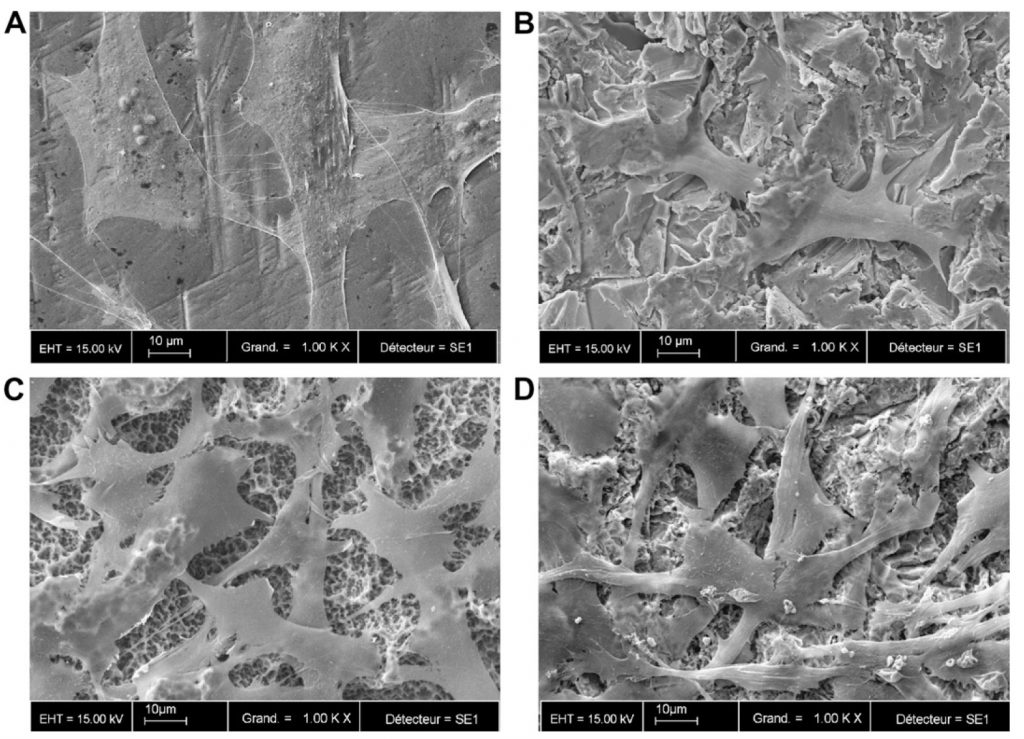
Figure 2: SEM micrographs of the MC3T3-E1 cells grown on different surfaces for 2 days. A – Smooth-Ti, B – Alumina-Ti, C – SLA, and D – BCP-Ti. Magnification X 1000. Bar = 10µm.
All surfaces were covered with a uniform layer of osteoblasts, even though they spread less on the rough titanium surfaces compared with the polished surface. Cells grown on the SLA surfaces developed more cytoplasmic extensions compared to Alumina-Ti and BCP-Ti.

The differentiation of the MC3T3-E1 cells grown on the different titanium surfaces and plastic surfaces (control) was assessed by alkaline phosphatase (ALP) activity assay (Figure 3) [22], ALP being an early marker for osteoblasts differentiation.
ALP activity increased with time, as expected, and reached its highest levels after 15 days.
After eight days of growing on the surfaces, no statistical difference
was found between the groups, though cells grown on SLA and BCP-Ti displayed slightly higher ALP levels, and Smooth-Ti and Alumina-Ti showed
slightly lower levels of ALP when compared to cells grown on plastic surfaces.
After 15 days, however, significant differences were observed between
Smooth-Ti and SLA, Alumina-Ti and SLA, and SLA and BCP-Ti.
After 21 days, no statistical differences were found between the groups.
After eight days of growing on the surfaces, no statistical difference was found between the groups, though cells grown on SLA and BCP-Ti displayed slightly higher ALP levels, and Smooth-Ti and Alumina-Ti showed slightly lower levels of ALP when compared to cells grown on plastic surfaces.

After 15 days, however, significant differences were observed between Smooth-Ti and SLA, Alumina-Ti and SLA, and SLA and BCP-Ti.
After 21 days, no statistical differences were found between the groups.
This study demonstrates that the roughness, surface processing technique, and blasting material have a significant effect on the adhesion and differentiation of osteoblasts. Rougher surfaces seem to contribute to the differentiation of the osteoblasts, and the contamination by the blasting particles, such as aluminum and carbon particles, also appear to affect the osteoblast’s differentiation and adhesion. Therefore, the BCP-grit blasting method, which combines the advantages of a rougher implant surface and less contamination by alumina and carbon, seems to exhibit the best combination of surface topography and chemistry out of all the other implants investigated.
For more info about BAS ™ Surface and the Scientific Research Documentation
In vitro & In vivo experiments please contact your local distributor.
Bibliography
[1] Davies JE. Mechanisms of endosseous integration. Int J Prosthodont 1998;11:391–401.
[2] Berglundh T, Abrahamsson I, Lang NP, Lindhe J. De novo alveolar bone formation adjacent to endosseous implants. Clin Oral Impl Res 2003;14:251–62.
[3] Albrektsson T, Wennerberg A. Oral implant surfaces. Part 2. A review focusing on clinical knowledge of different surfaces. Int J Prosthodont 2004;17:544–64.
[4] Esposito M, Coulthard P, Thomsen P, Worthington HV. The role of implant surface modifications, shape, and material on the success of osseointegrated dental implants. A Cochrane systematic review. Eur J Prosthodont Restor Dent 2005;13:15–31.
[5] Puleo DA, Thomas MV. Implant surfaces. Dent Clin N Am 2006;50:323–38.
[6] Cooper LF, Masuda T, Yliheikkila PK, Felton DA. Generalizations regarding the process and phenomenon of osseointegration. Part II. In vitro studies. Int J Oral Maxillofac Impl 1998;13:163–74.
[7] Anselme K. Osteoblast adhesion on biomaterials. Biomaterials 2000;21:667–81.
[8] Lauer G, Wiedmann-Al-Ahmad M, Otten JE, Hu¨bner U, Schmelzeisen R, Schilli W. The titanium surface texture affects adherence and growth of human gingival keratinocytes and human maxillar osteoblast-like cells in vitro. Biomaterials 2001;22:2799–809.
[9] Mustafa K, Wennerberg A, Wroblewski J, Hultenby K, Lopez BS, Arvidson K. Determining optimal surface roughness of TiO2 blasted titanium implant material for attachment, proliferation, and differentiation of cells derived from the human mandibular alveolar bone. Clin Oral Impl Res 2001;12:515–25.
[10] Ellingsen JE, Johansson CB, Wennerberg A, Holme´n A. Improved retention and bone-to-implant contact with fluoride-modified titanium implants. Int J Oral Maxillofac Impl 2004;19:659–66.
[11] Le Guehennec L, Soueidan A, Layrolle P, Amouriq Y. Surface treatments of titanium dental implants for rapid osseointegration. Dent Mater 2007;23:844–54.
[12] Esposito M, Hirsch JM, Lekholm U, Thomsen P. Biological factors contributing to failures of osseointegrated oral implants. (II). Etiopathogenesis. Eur J Oral Sci 1998;106:721–64.
[13] Sader MS, Balduino A, Soares Gde A, Borojevic R. Effect of three distinct treatments of titanium surface on osteoblast attachment, proliferation, and differentiation. Clin Oral Impl Res 2005;16:667–75.
[14] Citeau A, Guicheux J, Vinatier C, Layrolle P, Pilet P, Daculsi G. In vitro biological effects of titanium rough surface obtained by calcium phosphate grid blasting. Biomaterials 2005;26:157–65.
[15] Sanz A, Oyarzun A, Farias D, Diaz I. Experimental study of bone response to a new surface treatment of endosseous titanium implants. Impl Dent 2001;10:126–31.
[16] Novaes A, Souza S, de Oliveira P, Souza A. Histomorphometric analysis of the bone-implant contact obtained with 4 different implant surface treatments placed side by side in the dog mandible. Int J Oral Maxillofac Impl 2002;17:377–83.
[17] Buser D, Broggini N, Wieland M, Schenk RK, Denzer AJ, Cochran DL, Hoffmann B, Lussi A, Steinemann SG. Enhanced bone apposition to a chemically modified SLA titanium surface. J Dent Res 2004;83:529–33.
[18] Germanier Y, Tosatti S, Broggini N, Textor M, Buser D. Enhanced bone apposition around biofunctionalized sandblasted and acidetched titanium implant surfaces. A histomorphometric study in miniature pigs. Clin Oral Impl Res 2006;17:251–7.
[19] de Oliveira PT, Zalzal SF, Beloti MM, Rosa AL, Nanci A. Enhancement of in vitro osteogenesis on titanium by chemically produced nanotopography. J Biomed Mater Res A 2007;80:554–64.
[20] Zhao G, Raines AL, Wieland M, Schwartz Z, Boyan BD. Requirement for both micron- and submicron scale structure for synergistic responses of osteoblasts to substrate surface energy and topography. Biomaterials 2007;28:2821–9.
[21] Ahmad M, McCarthy MB, Gronowicz G. An in vitro model for mineralization of human osteoblast-like cells on implant materials. Biomaterials 1999;20:211–20.
[22] Le Guehennec L, Lopez-Heredia MC, Enkel B, Weiss P, Amourique Y, Layrolle P. Osteoblastic cell behaviour on different titanium implant surfaces. Acta Biomater. 2008;4:535-43.
Stay Safe. Wear a Mask!
The pink tissue versatile implant neck combines superior gingival aesthetics and high primary stability, improved placement, and temporization,
which is particularly beneficial in
aesthetically demanding cases.
The pink tissue versatile implant neck combines superior gingival aesthetics and high primary
stability, improved placement, and
temporization, which is particularly
beneficial in aesthetically
demanding cases.
The pink tissue versatile implant neck combines superior gingival aesthetics and high primary stability, improved placement, and temporization, which is particularly beneficial in aesthetically demanding cases.