Novel coatings to minimize bacterial adhesion and promote osteoblast activity for titanium implants.
The use of dental implants is a standard treatment for completely or partially edentulous patients. The artificial abutments reach the infected oral cavity by penetrating the oral mucosa, just as natural teeth do. The peri-implant tissue response appears to follow patterns similar to those of the periodontal tissues in a susceptible host when it is triggered by bacteria within the biofilms produced on implant surfaces, termed peri-implantitis (PI). PI is the most common cause of implant failure, and the most important target for novel biomaterials. The next generation of dental implants includes components that function to prevent PI, while maintaining the aesthetics of natural teeth [1, 2].
Dentalis® offers a novel coating on their implants that minimizes bacterial adhesion and corrosion on implant surfaces, without compromising the biocompatibility of titanium surfaces. Studies have proven the benefits of this coating as holding anti-bacterial and osseointegration properties. An in vitro assay showed that this coating decreases biofilm formation of P. gingivalis (a common PI-causing bacteria), while maintaining biocompatibility. The initial steps in stimulating bone formation and osseointegration are osteoblast adhesion and proliferation. When comparing coated vs uncoated surfaces, the researchers found that this coating increases both osteoblast adhesion and proliferation [3].
Bacterial Growth
After 4 h, the monomicrobial biofilm (Porphyromonas gingivalis) was less on the coated than the uncoated samples. The samples that were coated showed a biofilm coverage of 9.1% and 9.74%, respectively, whereas the uncoated samples showed a significantly higher biofilm coverage of 85.2% (p < 0.0001)
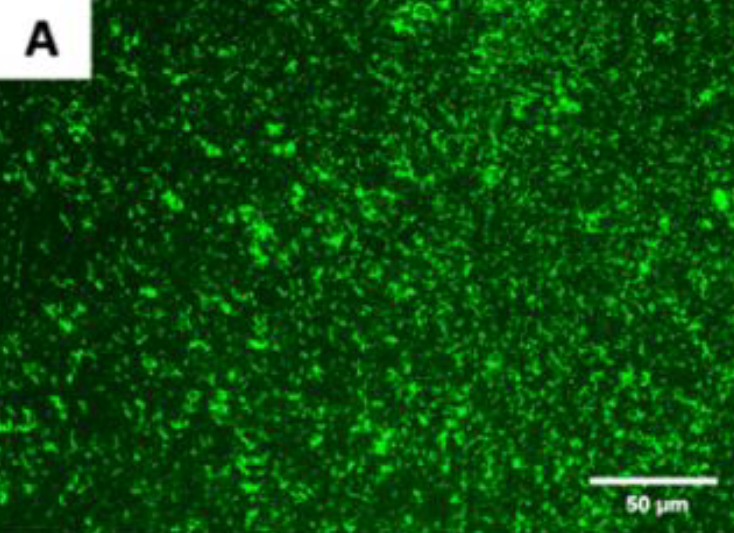
Figure A. Live fluorescence images of P. gingivalis cultured
for 4 h on the uncoated titanium disks.
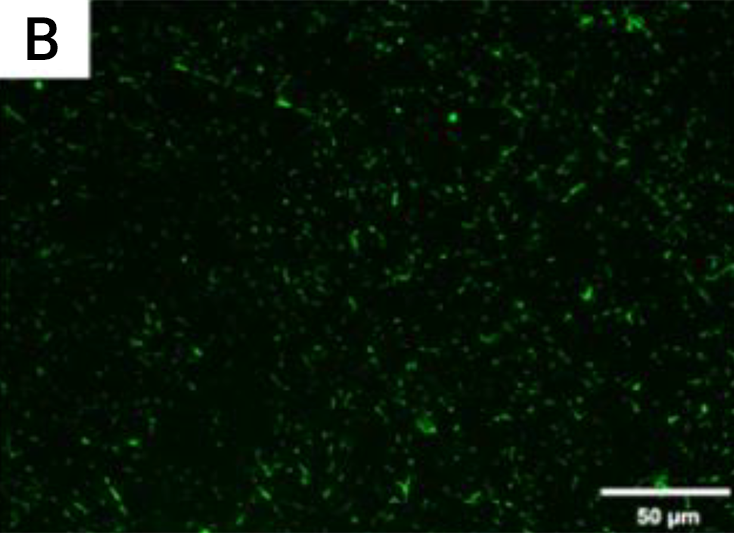
Figure B. Live fluorescence images of P. gingivalis cultured
for 4 h on the coated titanium disks.
The cultures were stained with SYTO® 9 to dye the living bacteria green.
(A) Uncoated; (B) coated;
The P. gingivalis coverage was calculated from at least 6 fields/disk at 10× g magnification using ImageJ software (F). Scale bar = 50 µm.
While the benefits of an anti-bacterial coating are quite obvious in that they prevent PI, the minimization of corrosion, abrasion, and ionization is equally important for the longevity of the implant as well as the patient’s comfort [4].
The metallic structures are exposed to a variety of intra-oral and extra-oral influences, which could lead to corrosion within the oral cavity. Saliva, exposure to acids in the diet, like from sour food and beverages, and exposure to acids produced by the oral biofilm are all linked to chemical corrosion. Additionally, mastication load and dental hygiene have an impact on metallic implants and cause mechanical-chemical corrosion. When two metallic surfaces with various electrochemical potentials interact in an electrolytic medium such as saliva, metal ions are released [5].
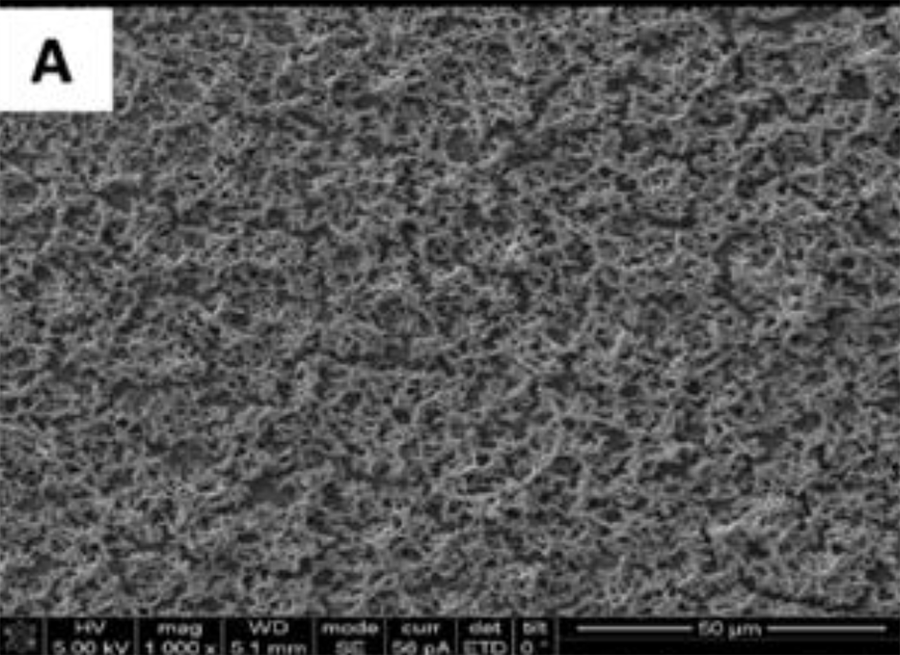
SEM adhesion of P. gingivalis after 24 h of cultivation on the
uncoated titanium disks. (A,)
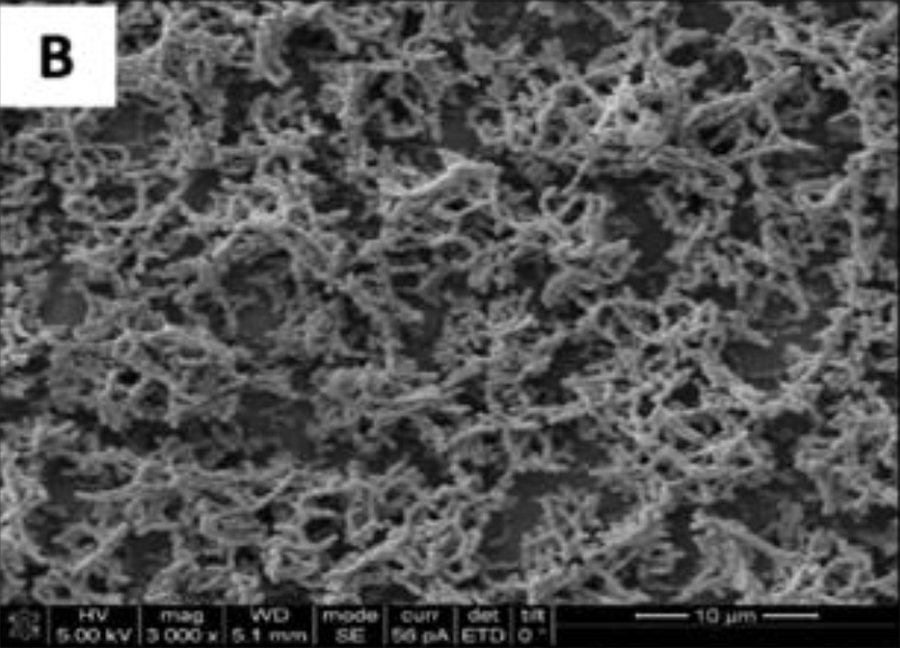
SEM adhesion of P. gingivalis after 24 h of cultivation on the
uncoated titanium disks. (,B)
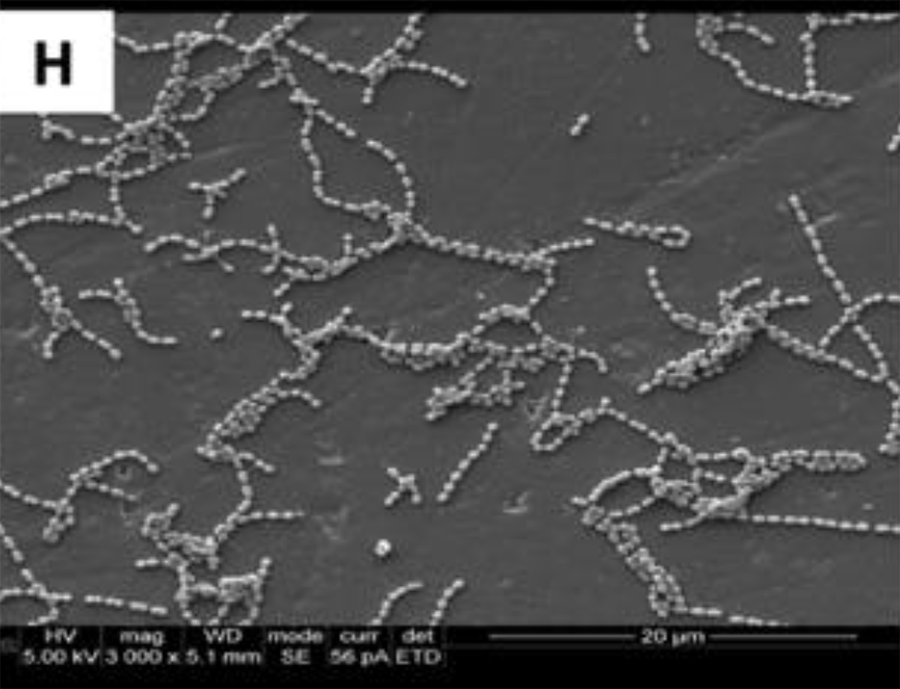
SEM adhesion of P. gingivalis after 24 h of cultivation on
the coated titanium disks H
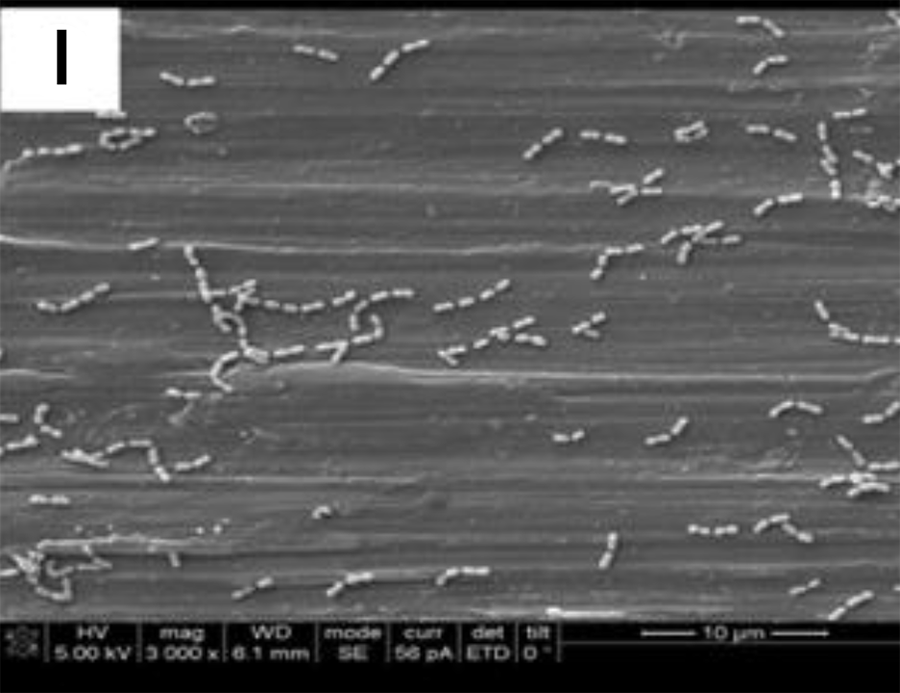
SEM adhesion of P. gingivalis after 24 h of cultivation on
the coated titanium disks H
SEM adhesion of P. gingivalis after 24 h of cultivation on the uncoated titanium disks. (A,B)
These data suggest that the coatings minimized the adhesion of a particular human pathogen associated with peri-implantitis. After 4 h of culture,
the fluorescence images in Figures A, and B demonstrated a higher P. gingivalis adhesion in the uncoated group than on the coated groups.
The SEM images show the results from the live assays, demonstrating a decrease in the number of bacteria adhesions
on the coated groups for P. gingivalis after 24 h of culture (Figure 2).
Ionization of dental alloys can cause allergic reactions, resulting in chronic inflammation at the peri-implant site. All metallic implant parts eventually release ions into the environment, which in certain people can result in allergic responses. It is especially problematic for patients with hypersensitivity to metallic ingredients. The three metals that are most frequently reported to cause allergies can be found in the implant substrate materials: nickel (Ni), cobalt (Co), and chromium (Cr). An allergic reaction is less likely when this biocompatible and inert coating is applied because it serves as a barrier to such ion release. In vitro assays have shown that Co, Cr, and Ni ion concentrations were below detectable limits of coated in saliva solution, providing evidence that this coating will minimize the risk of an allergic reaction in clinical practice [6]. Similarly, a study showed that the coating minimized abrasion (another cause of implant failure) and had high wear resistance. In fact, the researchers found that coated components have 38% of the wear rate of the uncoated components, and this was attributed to the sophisticated articulating surface quality and high wettability, which reduces friction [7].
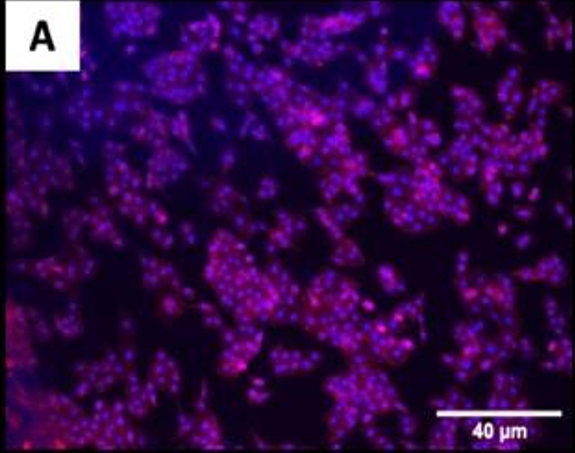
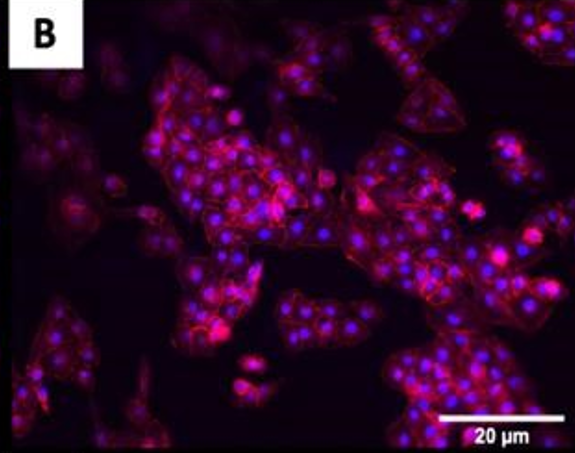
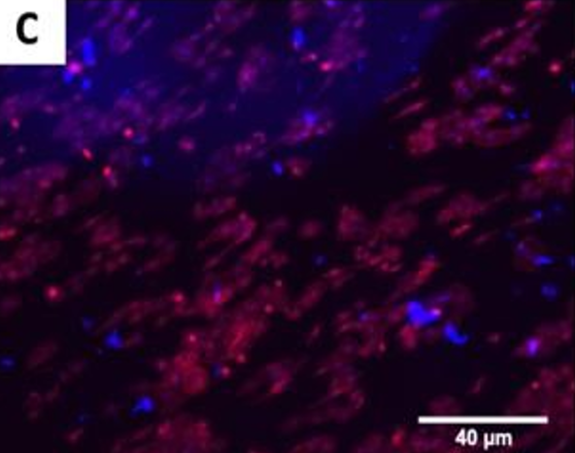
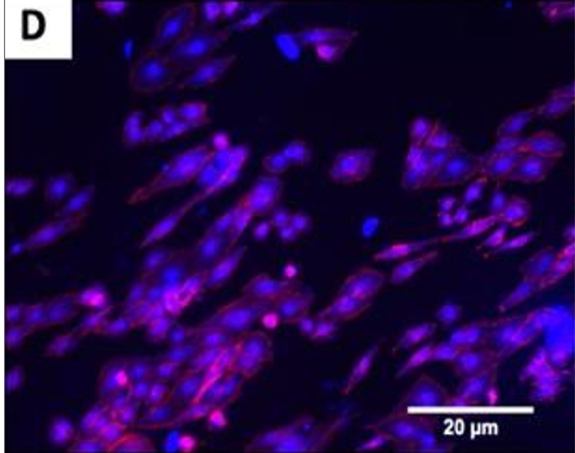
The fluorescence images demonstrated that cells adhered and covered the surface of the disk after 24 h in culture.
A,B Uncoated; C, D QSiC-coated
Additionally, the cell morphology was similar for cells cultured on the coated and uncoated samples, where the Nhosts appear to be oval-shaped and flattened on the surface.
Dentalis’ antibacterial, osseointegrative, and biocompatible coating provides for a long-lasting, esthetic, and functional implant that reduces the risk of peri-implantitis, corrosion, and allergic reaction.
References
- Klinge, B., M. Hultin, and T. Berglundh, Peri-implantitis. Dental Clinics, 2005. 49(3): p. 661-676.
- Schwarz, F., et al., Peri‐implantitis. Journal of clinical periodontology, 2018. 45: p. S246-S266.
- Camargo, S.E., et al., Novel coatings to minimize bacterial adhesion and promote osteoblast activity for titanium implants. Journal of Functional Biomaterials, 2020. 11(2): p. 42.
- Bhola, R., et al., Corrosion in titanium dental implants/prostheses–a review. Trends Biomater Artif Organs, 2011. 25(1): p. 34-46.
- Arakelyan, M., et al., Minimization of Adverse Effects Associated with Dental Alloys. Materials, 2022. 15(21): p. 7476.
- Thomas, P., et al., Influence of surface coating on metal ion release: evaluation in patients with metal allergy. Orthopedics, 2016. 39(3): p. S24-S30.
- van Hove, R.P., et al., Titanium-nitride coating of orthopaedic implants: a review of the literature. BioMed research international, 2015. 2015.
Dentalis Gold tissue-level implants make simpler and more predictable treatments available, which can significantly increase patient acceptance of implant treatment.
For more info about the new novel coatings to minimize bacterial and how to avoid peri-implantitis Send us a message
The coated neck of the implant is antibacterial and prevents the formation of biofilms.
State-of-the-art nitriding of titanium allows increases in the depth of nitrogen diffusion, therefore increasing soft tissue adhesion.
The attachment of soft tissue, peri-implant mucosa, surrounding a dental implant creates a seal and is composed of epithelial and connective tissue.
The tissue-level design provides for a more stable seal.

