Dentalis Nano Level Surface
Dentalis Nano Level Surface
The issue of bone-implant surface nanoroughness has received growing attention in the field of implant surface interaction in the last few years [1,2], as implant manufacturers – including dental implants – attempt to increase osseointegration via adjustments to surface topographies and implant chemistries [3]. Increased osteoconductivity and biocompatibility have been found to lead to better implant loading, as well as increased ossification expected even in areas with low bone mass and quality. [4,5]
In addition to the roughness of the implant surface, chemical modifications to the surface can also contribute to better bone creation and healing [2,6], as was previously shown in vivo and in vitro [7-11]. Presently, most implants are coated or saturated with crystals such as hydroxyapatite [12,13] or calcium phosphate [14-17].
Despite the importance of these chemical modifications, it is generally agreed upon that the texture of the implant surface is a significant factor in bone formation. [4,5] When compared to smooth-surface implants, creating
surface roughness at the micrometer scale results in earlier fixation of the implants [4,5], seemingly due to improved protein absorption, cell adhesion, proliferation, and differentiation [1], though the role of roughness at the nanometer scale is still unclear.
The interactions between hosts and “nano” implants are being actively
studied and seem highly promising [12,13,15,16,18-20]; however, different chemical coating options make comparisons between different implants
much harder, as they often contribute to the shape and roughness of the implant surface.
A recent study compared an implant infused with small amounts of calcium (Ca) and phosphate (P) on a surface with nanoroughness qualities (CaP implant) to a control implant with a smoother surface on the nanoscale, processed through alumina-blasting/dual acid etching, and showed increased biomechanical fixation in the CaP implant. Another study compared these two implants in terms of bone cell activity [21].








Despite the importance of these chemical modifications, it is generally agreed upon that the texture of the implant surface is a significant factor in bone formation. [4,5] When compared to smooth-surface implants, creating
surface roughness at the micrometer scale results in earlier fixation of the implants [4,5], seemingly due to improved protein absorption, cell adhesion, proliferation, and differentiation [1], though the role of roughness at the nanometer scale is still unclear.
The interactions between hosts and “nano” implants are being actively
studied and seem highly promising [12,13,15,16,18-20]; however, different chemical coating options make comparisons between different implants
much harder, as they often contribute to the shape and roughness of the implant surface.
A recent study compared an implant infused with small amounts of calcium (Ca) and phosphate (P) on a surface with nanoroughness qualities (CaP implant) to a control implant with a smoother surface on the nanoscale, processed through alumina-blasting/dual acid etching, and showed increased biomechanical fixation in the CaP implant. Another study compared these two implants in terms of bone cell activity [21].








Table I presents the differences in surface qualities as measured by a scanning electron microscope, and the most significant difference between the two implants appears to be the distance between the peaks on the surface – the CaP surface demonstrated much closer peaks than the control.
Quantification of the remaining blasting media on the implant surface is shown in Table II. Due to the sandblasting technique used in the control implant, residues of aluminum (Al) and silicon (Si) contaminated the implant surface.
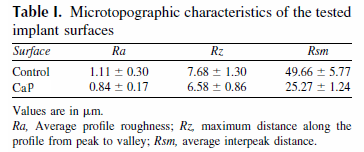
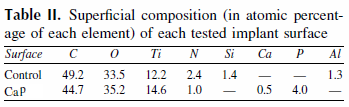
Figure 1 shows SEM analysis of the surfaces of both implants.
A different topography can be observed on the micrometric scale,
with similar peak heights but shorter distances between the
peaks in the CaP implant.
The nanometric level shows significant differences between
the samples: the surface of the control is almost flat, while the CaP
surface demonstrates homogeneous nano-roughness.
In addition, the calcium phosphate nanocrystals could not be
observed, even in the highest magnification.
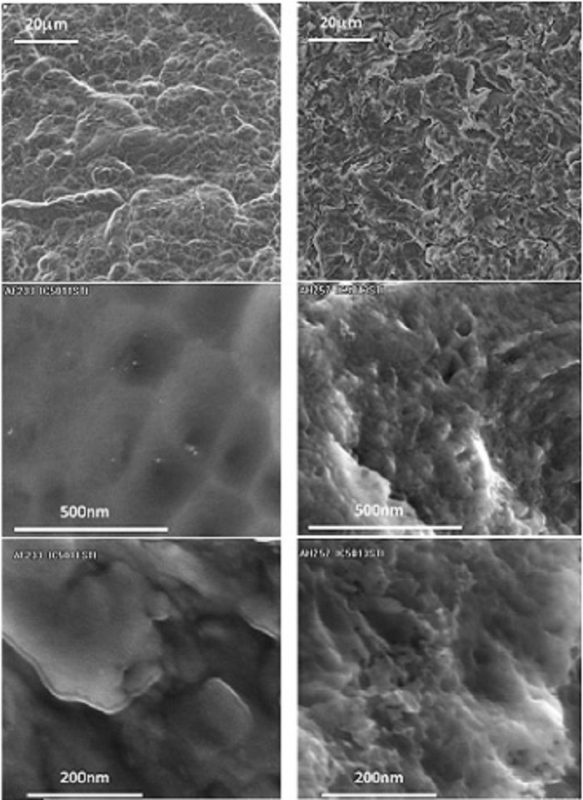
Figure 1 shows SEM analysis of the surfaces of both implants. A different topography can be observed on the micrometric scale, with similar peak heights but shorter distances between the peaks in the CaP implant.
The nanometric level shows significant differences between the samples: the surface of the control is almost flat, while the CaP surface demonstrates homogeneous nano-roughness.
In addition, the calcium phosphate nanocrystals could not be observed, even in the highest magnification.

Fig. 1 Scanning electron microscope (SEM) analysis of the surfaces of control and CaP implants. Magnifications are indicated on the left.
Both implants enabled osteoblast adhesion and growth, without signs of cytotoxicity.
Fig. 2 shows the adhesion of osteoblasts to the surfaces of the implants. In both implants, the pattern is similar:
after six hours, some cells are round, but some flatten and begin to present pseudopods and vesicles, indicating metabolic activity and the
beginning of adhesion.
After 24 hours, most cells have flattened on the surface, and after 72 hours, all the cells are flat and mimic the surface topography, creating a veil
of osteoblasts on the surface of the implants.
Despite the similarities, the process accelerated on the CaP surface, and the osteoblasts showed more
vesicles and pseudopods after only six hours, which is much less time than the control.
Fig 2. SEM analysis of Osteoblast adhesion to the surfaces of the implants. Magnifications and time are indicated on the left.
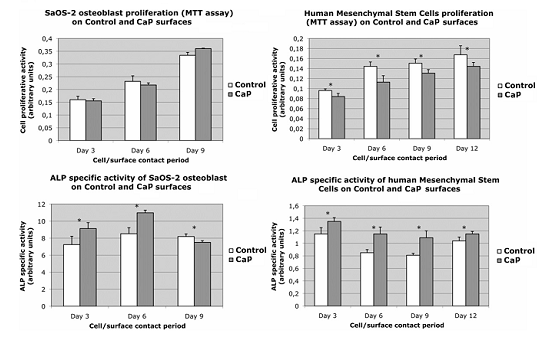
Assessment of the proliferation and ALP activity of osteoblasts
is presented in Fig.3.
While the proliferation of osteoblasts on both surfaces was not significantly different, ALP-specific activity showed significantly higher values in the osteoblasts in contact with the CaP implant surface.
The proliferation of osteoblast progenitors, human mesenchymal stem
cells (hMSCs), was lower on the CaP surface, but the ALP-specific activity
was once again higher in these cells
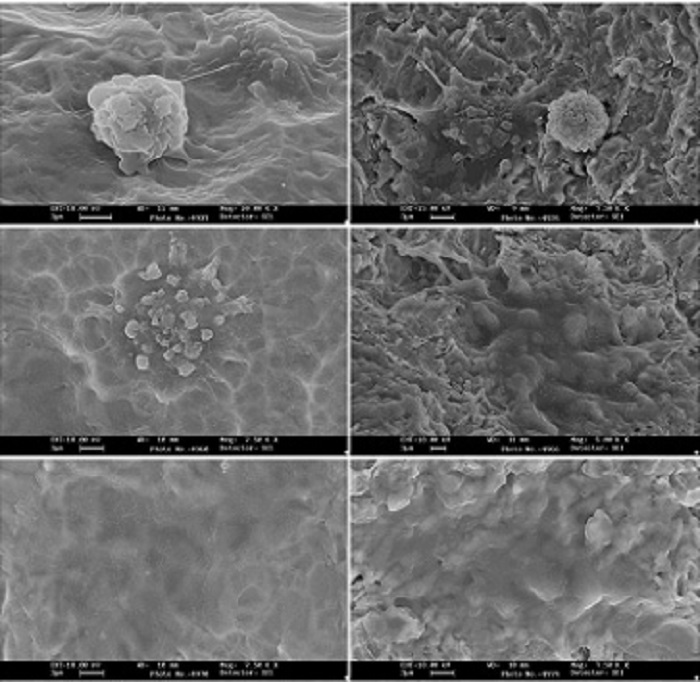
Fig. 3 Proliferative activity assay (MTT assay) and alkaline phosphatase
(ALP) the specific activity of osteoblasts and human mesenchymal stem
cells after 3,6,9 and 12 days in contact with the surfaces of both implants.
* values are significantly different (P < 0.01).
In conclusion, the CaP implants showed positive results correlated with cell surface nanoroughness compared to the control implant,
and the low infusion resulted in nanocrystals that could not be detected with SEM even on high magnifications.
The adhesion of osteoblasts to the surface of the CaP implant seems to appear earlier in “nano” implants than on the control implant,
and despite the fact that no changes were observed in the number of osteoblasts on both surfaces and lower hMSCs on the CaP surface,
ALP activity – a marker for osteoblasts’ activity – was higher on the CaP surface in both cell types.
For more info about BAS ™ Surface and the Scientific Research
Documentation In vitro & In vivo experiments, please
contact your local distributor or
email us:
[email protected]
Bibliography:
1. Mendonca G, Mendonca DB, Aragao FJ, Cooper LF. Advancing dental implant surface technology—from micro- to nanotopography. Biomaterials 2008;29:3822-35.
2. Coelho PG, Granjeiro JM, Romanos GE, Suzuki M, Silva NR, Cardaropoli G, et al. Basic research methods and current trends of dental implant surfaces. J Biomed Mater Res B Appl Biomater 2009;88:579-96.
3. Albrektsson T, Sennerby L, Wennerberg A. State of the art of oral implants. Periodontol 2000 2008;47:15-26.
4. Albrektsson T, Wennerberg A. Oral implant surfaces: part 1—review focusing on topographic and chemical properties of different surfaces and in vivo responses to them. Int J Prosthodont 2004;17:536-43.
5. Albrektsson T, Wennerberg A. Oral implant surfaces: part 2—review focusing on clinical knowledge of different surfaces. Int J Prosthodont 2004;17:544-64.
6. Kang BS, Sul YT, Oh SJ, Lee HJ, Albrektsson T. XPS, AES and SEM analysis of recent dental implants. Acta Biomater 2009; 5(6):2222-9.
7. Lamolle SF, Monjo M, Rubert M, Haugen HJ, Lyngstadaas SP, Ellingsen JE. The effect of hydrofluoric acid treatment of titanium surface on nanostructural and chemical changes and the growth of MC3T3-E1 cells. Biomaterials 2009;30:736-42.
8. Ellingsen JE, Johansson CB, Wennerberg A, Holmen A. Improved retention and bone-to-implant contact with fluoride-modified titanium implants. Int J Oral Maxillofac Implants 2004; 19:659-66.
9. Monjo M, Lamolle SF, Lyngstadaas SP, Ronold HJ, Ellingsen JE. In vivo expression of osteogenic markers and bone mineral density at the surface of fluoride-modified titanium implants. Biomaterials 2008;29:3771-80.
10. Sul YT, Johansson C, Byon E, Albrektsson T. The bone response of oxidized bioactive and non bioactive titanium implants. Biomaterials 2005;26:6720-30.
11. Sul YT, Kang BS, Johansson C, Um HS, Park CJ, Albrektsson T. The roles of surface chemistry and topography in the strength and rate of osseointegration of titanium implants in bone. J Biomed Mater Res 2009;89(4):942-50.
12. Meirelles L, Albrektsson T, Kjellin P, Arvidsson A, Franke-Stenport V, Andersson M, et al. Bone reaction to nano-hydroxyapatite modified titanium implants placed in a gap-healing model. J Biomed Mater Res 2008;87:624-31.
13. Meirelles L, Arvidsson A, Andersson M, Kjellin P, Albrektsson T, Wennerberg A. Nano hydroxyapatite structures influence early bone formation. J Biomed Mater Res A 2008;87:299-307.
14. Yang Y, Kim KH, Ong JL. A review on calcium phosphate coatings produced using a sputtering process—an alternative to plasma spraying. Biomaterials 2005;26:327-37.
15. Coelho PG, Cardaropoli G, Suzuki M, Lemons JE. Early healing of nano thickness bioceramic coatings on dental implants. An experimental study in dogs. J Biomed Mater Res B Appl Biomater 2009;88:387-93.
16. Coelho PG, Cardaropoli G, Suzuki M, Lemons JE. Histomorphometric evaluation of a nano thickness bioceramic deposition on endosseous implants: a study in dogs. Clin Implant Dent Relat Res 2008; In Press.
17. Granato R, Marin C, Suzuki M, Gil JN, Janal MN, Coelho PG. Biomechanical and histomorphometric evaluation of a thin ion beam bioceramic deposition on plateau root form implants an experimental study in dogs. J Biomed Mater Res B Appl Biomater 2009;90(1):396-403.
18. Meirelles L, Currie F, Jacobsson M, Albrektsson T, Wennerberg A. The effect of chemical and nanotopographical modifications on the early stages of osseointegration. Int J Oral Maxillofac Implants 2008;23:641-7.
19. Coelho PG, Lemons JE. Physico/chemical characterization and in vivo evaluation of nano thickness bioceramic depositions on alumina-blasted/acid-etched Ti-6Al-4V implant surfaces. J Biomed Mater Res 2009;90(2):351-61.
20. Marin C, Granato R, Suzuki M, Gil JN, Piattelli A, Coelho PG. Removal torque and histomorphometric evaluation of bioceramic grit-blasted/acid-etched and dual acid-etched implant surfaces: an experimental study in dogs. J Periodontol 2008;79:1942-9.
21. Bucci-Sabattini V,Cassinelli C, Coelho P.G, DDS, Minnici A, Trani A, Dohan Ehrenfest. Effect of titanium implant surface nanoroughness and calcium phosphate low impregnation on bone cell activity in vitro. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2010 Feb;109(2):217-24.
Stay Safe. Wear a Mask!
The combination of innovative surface technology with 344% stronger bone reduces marginal bone loss and provides for a higher BIC%, decreasing
the risk of peri-implant disease. The enhanced deep thread simplifies the insertion and allows for high primary stability.
This is the first dental implant to combine the concept of tissue-level
implants with an anti-bacterial coating on the implant neck, preventing
peri-implant disease and providing predictable results you can trust.
The combination of innovative surface technology with 344% stronger bone reduces marginal bone loss and provides for a higher BIC%, decreasing
the risk of peri-implant disease. The enhanced deep thread simplifies the insertion and allows for high primary stability.
This is the first dental implant to combine the concept of tissue-level implants with an anti-bacterial coating on the implant neck,
preventing peri-implant disease and
providing predictable results
you can trust.
The combination of innovative surface technology with 344% stronger bone reduces marginal bone loss and provides for a higher BIC%, decreasing
the risk of peri-implant disease. The enhanced deep thread simplifies the insertion and allows for high primary stability.
This is the first dental implant to combine the concept of tissue-level implants with an anti-bacterial coating on the implant neck, preventing peri-implant disease and providing predictable results you can trust.